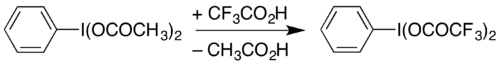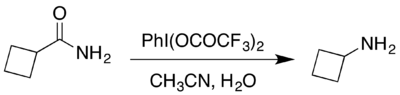(Bis(trifluoroacetoxy)iodo)benzene
(Bis(trifluoroacetoxy)iodo)benzene, C
6H
5I(OCOCF
3)
2, is a hypervalent iodine compound used as a reagent in organic chemistry. It can be used to carry out the Hofmann rearrangement under acidic conditions.[1]
iodo)benzene.png.webp) | |
iodo)benzene-3D-balls.png.webp) | |
| Names | |
|---|---|
| IUPAC name
phenyl{bis[(trifluoroacetyl)oxy]}-λ3-iodane | |
| Other names
Phenyliodine bis(trifluoroacetate); PIFA | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.018.462 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H5F6IO4 | |
| Molar mass | 430.041 g·mol−1 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
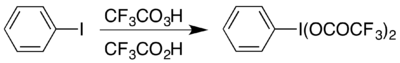
The syntheses of all aryl hypervalent iodine compounds start from iodobenzene. The compound can be prepared by reaction of iodobenzene with a mixture of trifluoroperacetic acid and trifluoroacetic acid in a method analogous to the synthesis of (diacetoxyiodo)benzene:[1]
It can also be prepared by dissolving diacetoxyiodobenzene (a commercially-available compound) with heating in trifluoroacetic acid:[2]
Uses
It also brings around the conversion of a hydrazone to a diazo compound, for example in the diazo-thioketone coupling. It also converts thioacetals to their parent carbonyl compounds.
Hofmann rearrangement
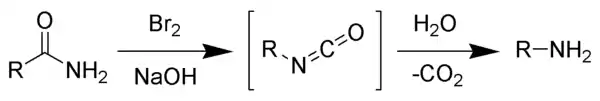
The Hofmann rearrangement is a decarbonylation reaction whereby an amide is converted to an amine by way of an isocyanate intermediate. It is usually carried out under strongly basic conditions.[3][4]
The reaction can also be carried out under mildly acidic conditions by way of the same intermediate using a hypervalent iodine compound in aqueous solution.[1] An example published in Organic Syntheses is the conversion of cyclobutanecarboxamide, easily synthesized from cyclobutylcarboxylic acid, to cyclobutylamine.[2] The primary amine is initially present as its trifluoroacetate salt, which can be converted to the hydrochloride salt to facilitate product purification.[1][2]
References
- Aubé, Jeffrey; Fehl, Charlie; Liu, Ruzhang; McLeod, Michael C.; Motiwala, Hashim F. (1993). "6.15 Hofmann, Curtius, Schmidt, Lossen, and Related Reactions". Heteroatom Manipulations. Comprehensive Organic Synthesis II. 6. pp. 598–635. doi:10.1016/B978-0-08-097742-3.00623-6. ISBN 9780080977430.
- Almond, M. R.; Stimmel, J. B.; Thompson, E. A.; Loudon, G. M. (1988). "Hofmann Rearrangement Under Mildly Acidic Conditions Using [I,I-Bis(Trifluoroacetoxy)]Iodobenzene: Cyclobutylamine Hydrochloride from Cyclobutanecarboxamide". Organic Syntheses. 66: 132. doi:10.15227/orgsyn.066.0132.; Collective Volume, 8, p. 132
- Wallis, Everett S.; Lane, John F. (1946). "The Hofmann Reaction". Organic Reactions. 3 (7): 267–306. doi:10.1002/0471264180.or003.07.
- Surrey, Alexander R. (1961). "Hofmann Reaction". Name Reactions in Organic Chemistry (2nd ed.). Academic Press. pp. 134–136. ISBN 9781483258683.