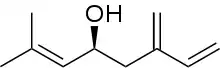
(S)-Ipsdienol
(S)-Ipsdienol is a terpene alcohol. It is one of the major aggregation pheromones of the bark beetle. It was first identified from Ips confusus, in which it is believed to be a principle sex attractant.[1] It is suggested that the compound plays a role in interspecies communication between Ips latidens and Ips ini, facilitating reductions in competition for breeding material and/or mating interference.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(4S)-2-methyl-6-methylideneocta-2,7-dien-4-ol | |
| Other names
(S)-(+)-Ipsdienol, 2-Methyl-6-methylene-2,7-octadiene-4-ol, Ipsdienol | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.128.974 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C10H16O | |
| Molar mass | 152.237 g·mol−1 |
| Appearance | Colorless |
| Hazards | |
| Flash point | 87 °C (189 °F; 360 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
The compound has been synthesized from D-mannitol.[3] Alternative syntheses were realized through the asymmetric isoprenylation of correspondent aldehyde (prenal)[4] and alcohol (prenol).[5] Chiral resolution of racemic precursor has been found[6][7] to provide both enantiomers of ipsdienol in high enentiomeric purity and in preparative scale.
References
- Silverstein, Robert M.; Rodin, J. Otto; Wood, David L. (October 1966). "Sex Attractants in Frass Produced by Male Ips confusus in Ponderosa Pine". Science. 154 (3748): 509–510. Bibcode:1966Sci...154..509S. doi:10.1126/science.154.3748.509 (inactive 2021-01-17). JSTOR 1720044.CS1 maint: DOI inactive as of January 2021 (link)
- Miller, Daniel R.; Borden, John H.; King, G. G. S.; Slessor, Keith N. (1 August 1991). "Ipsenol: an aggregation pheromone for Ips latidens (Leconte) (Coleoptera: Scolytidae)". Journal of Chemical Ecology. 17 (8): 1517–1527. doi:10.1007/BF00984685. PMID 24257877. S2CID 22337300.
- Hanessian, Stephen (1983). Total Synthesis of Natural Products: The 'Chiron' Approach. Pergamon press. p. 63. ISBN 978-0-08-029247-2.
- Zhang, Yu‐Long; He, Bo‐Jun; Xie, Yi‐Wen; Wang, Yu‐Hao; Wang, Yi‐Long; Shen, Yong‐Cun; Huang, Yi‐Yong (2019). "Enantioselective Isoprenylboration Reaction of Aldehydes Catalyzed by a Chiral Phosphoric Acid". Advanced Synthesis & Catalysis. 361 (13): 3074–3079. doi:10.1002/adsc.201900203.
- Xiang, Ming; Luo, Guoshun; Wang, Yuankai; Krische, Michael J. (2019). "Enantioselective iridium-catalyzed carbonyl isoprenylation via alcohol-mediated hydrogen transfer". Chemical Communications. 55 (7): 981–984. doi:10.1039/C8CC09706B. PMC 6339811. PMID 30608076.
- Kovalenko, V. N.; Prokhorevich, K. N. (2016). "Improved synthesis of optically active ipsdienol". Russian Journal of Organic Chemistry. 52 (5): 757–758. doi:10.1134/S1070428016050250. S2CID 99770908.
- Kovalenko, V. N.; Matyushenkov, E. A. (2012). "Stereoselective synthesis of (R)- and (S)-Ipsdienols, pheromone components of bark beetles of the Ips family". Russian Journal of Organic Chemistry. 48 (9): 1168–1172. doi:10.1134/S1070428012090035. S2CID 84179515.