1,1'-Azobis-1,2,3-triazole
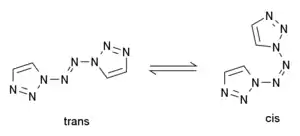
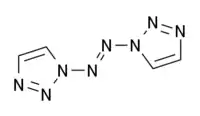
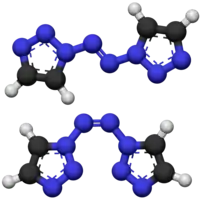
1,1′-Azobis-1,2,3-triazole is a moderately explosive but comparatively stable chemical compound which contains a long continuous chain of nitrogen atoms, with an unbroken chain of eight nitrogen atoms cyclised into two 1,2,3-triazole rings. It is stable up to 194 °C. The compound exhibits cis–trans isomerism at the central azo group: the trans isomer is more stable and is yellow, while the cis isomer is less stable and is blue. The two rings are aromatic and form a conjugated system with the azo linkage. This chromophore allows the trans compound to be isomerised to the cis when treated with an appropriate wavelength of ultraviolet light.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1′-Azobis-1,2,3-triazole | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C4H4N8 | |
| Molar mass | 164.132 g·mol−1 |
| Hazards | |
| Main hazards | Explosive |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Related compounds
In 2011, azobis(tetrazole) was prepared by Klapötke and Piercey which has a ten-nitrogen chain.[2] The record was later taken by a N11 chain compound synthesized by a group of Chinese researchers.[3] A branched chain N11 system has also been reported as part of an unstable but highly nitrogen rich azidotetrazole derivative with formula C2N14.[4]
See also
- HBT (explosive)
- G2ZT
- Diazene
References
- Li, Y. C.; Qi, C.; Li, S. H.; Zhang, H. J.; Sun, C. H.; Yu, Y. Z.; Pang, S. P. (2010). "1,1′-Azobis-1,2,3-triazole: A High-Nitrogen Compound with Stable N8 Structure and Photochromism". Journal of the American Chemical Society. 132 (35): 12172–3. doi:10.1021/ja103525v. PMID 20715773.
- Klapötke, Thomas M.; Piercey, Davin G. (2011). "1,1′-Azobis(tetrazole): A Highly Energetic Nitrogen-Rich Compound with a N10 Chain". Inorg. Chem. 50 (7): 2732–2734. doi:10.1021/ic200071q. PMID 21384800.
- Tang, Y.; Yang, H.; Wu, B.; Ju, X.; Lu, C.; Cheng, G. (2013). "Synthesis and Characterization of a Stable, Catenated N11Energetic Salt". Angewandte Chemie International Edition. 52 (18): 4875–7. doi:10.1002/anie.201300117. PMID 23554233.
- Klapötke, T. M.; Krumm, B.; Martin, F. A.; Stierstorfer, J. R. (2012). "New Azidotetrazoles: Structurally Interesting and Extremely Sensitive". Chemistry: An Asian Journal. 7 (1): 214–224. doi:10.1002/asia.201100632. PMID 22069147.