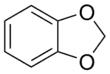
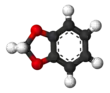
1,3-Benzodioxole
1,3-Benzodioxole (1,2-methylenedioxybenzene) is an organic compound with the formula C6H4O2CH2. The compound is classified as benzene derivative and a heterocyclic compound containing the methylenedioxy functional group. It is a colorless liquid.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2H-1,3-Benzodioxole | |||
| Other names
1,3-Benzodioxole Benzo[d][1,3]dioxole 1,2-[Methylenebis(oxy)]benzene 1,2-Methylenedioxybenzene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 115506 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.448 | ||
| EC Number |
| ||
| MeSH | 1,3-Benzodioxole | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1993 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6O2 | |||
| Molar mass | 122.123 g·mol−1 | ||
| Density | 1.064 g cm−3 | ||
| Boiling point | 172–173 °C (342–343 °F; 445–446 K) | ||
| log P | 2.08 | ||
| Vapor pressure | 1.6 kPa | ||
| Thermochemistry | |||
Std enthalpy of combustion (ΔcH⦵298) |
-3.428 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
| H302, H332 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 61 °C (142 °F; 334 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Although benzodioxole is not particularly important, many related compounds containing the methylenedioxyphenyl group are bioactive, and thus are found in pesticides and pharmaceuticals.[1]
Preparation
1,3-Benzodioxole can be synthesized from catechol with disubstituted halomethanes.[2][3]
See also
References
- Murray, M., "Mechanisms of inhibitory and regulatory effects of methylenedioxyphenyl compounds on cytochrome P450-dependent drug oxidation", Curr. Drug Metab. 2000, volume 1, 67-84. doi:10.2174/1389200003339270
- Bonthrone, W. & Cornforth, J. (1969). "The methylenation of catechols". Journal of the Chemical Society (9): 1202–1204. doi:10.1039/J39690001202. Retrieved 28 December 2013.
- Fujita, Harushige; Yamashita & Masataro (1973). "The Methylenation of Several Allylbenzene-1,2-diol Derivatives in Aprotic Polar Solvents". Bulletin of the Chemical Society of Japan. 46 (11): 3553–3554. doi:10.1246/bcsj.46.3553. Retrieved 27 December 2013.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.