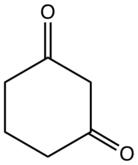
1,3-Cyclohexanedione
1,3-Cyclohexanedione is an organic compound with the formula (CH2)4(CO)2. It is one of three isomeric cyclohexanediones. It is a colorless compound that occurs naturally. It is the substrate for cyclohexanedione hydrolase. The compound exists mainly as the enol tautomer.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexane-1,3-dione | |
| Other names
CHD, dihydroresorcinol | |
| Identifiers | |
3D model (JSmol) |
|
| 385888 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.255 |
| EC Number |
|
| 200899 | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H8O2 | |
| Molar mass | 112.128 g·mol−1 |
| Appearance | Colorless or white solid |
| Density | 1.0861 g/cm3 |
| Melting point | 105.5 °C (221.9 °F; 378.6 K) |
| Acidity (pKa) | 5.20 (H2O)[1] |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
| H302, H318, H412 | |
| P264, P270, P273, P280, P301+312, P305+351+338, P310, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis, structure, and reactivity
1,3-Cyclohexanedione is produced by semi-hydrogenation of resorcinol:[3]
- C6H4(OH)2 + H2 → C6H8O2
1,3-Cyclohexanedione exists in solution as the enol. It reacts under acid catalysis with alcohols to 3-alkoxyenones.[2]
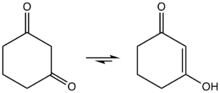 Enolization of 1,3-cyclohexanedione.
Enolization of 1,3-cyclohexanedione.
Its pKa is 5.26. Treatment of the sodium salt of the enolate with methyl iodide gives 2-methyl-1,3-cyclohexanedione, which despite the name, also exists predominantly as the enol.[3]
Derivatives
Dimedone, 5,5-dimethyl-1,3-cyclohexanedione is a well established reagent.
Several herbicides are formal derivatives of 1,3-cyclohexanedione. Examples of commercial products include cycloxydim, clethodim, tralkoxydim, butroxydim, profoxydim, mesotrione and quizalofop-P-ethyl.[4]
References
- Terasawa, Tadao; Okada, Toshihiko (1977). "Novel heterocyclic synthons. Synthesis and properties of thia- and oxacyclohexane-3,5-diones". J. Org. Chem. 42 (7): 1163–1169. doi:10.1021/jo00427a014.
- Guppi, Sanjeeva Rao; O'Doherty, George A. (2008). "1,3-Cyclohexadiene". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00921.
- Mekler, A. B.; Ramachandran, S.; Swaminathan, S.; Newman, Melvin S. (1961). "Methyl-1,3-Cyclohexanedione". Org. Synth. 41: 56. doi:10.15227/orgsyn.041.0056.
- Keith G. Watson (2011). "Cyclohexane-1,3-dione Oxime Ether Grass-Specific Herbicides and the Discovery of Butroxydim". Aust. J. Chem. 64: 367–372. doi:10.1071/CH10366.