2-Deoxy-D-glucose
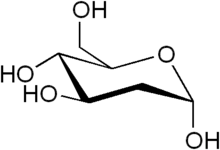
2-Deoxy-d-glucose is a glucose molecule which has the 2-hydroxyl group replaced by hydrogen, so that it cannot undergo further glycolysis. As such; it acts to competitively inhibit the production of glucose-6-phosphate from glucose at the phosphoglucoisomerase level (step 2 of glycolysis).[2] In most cells, glucose hexokinase phosphorylates 2-deoxyglucose, trapping the product 2-deoxyglucose-6-phosphate intracellularly (with exception of liver and kidney); thus, labelled forms of 2-deoxyglucose serve as a good marker for tissue glucose uptake and hexokinase activity. Many cancers have elevated glucose uptake and hexokinase levels. 2-Deoxyglucose labeled with tritium or carbon-14 has been a popular ligand for laboratory research in animal models, where distribution is assessed by tissue-slicing followed by autoradiography, sometimes in tandem with either conventional or electron microscopy.
 | |
| Names | |
|---|---|
| IUPAC name
(4R,5S,6R)-6-(hydroxymethyl)oxane-2,4,5-triol | |
| Other names
2-Deoxyglucose 2-Deoxy-d-mannose 2-Deoxy-d-arabino-hexose 2-DG | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H12O5 | |
| Molar mass | 164.16 g/mol |
| Melting point | 142 to 144 °C (288 to 291 °F; 415 to 417 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-DG is uptaken by the glucose transporters of the cell. Therefore, cells with higher glucose uptake, for example tumor cells, have also a higher uptake of 2-DG. Since 2-DG hampers cell growth, its use as a tumor therapeutic has been suggested, and in fact, 2-DG is in clinical trials. [3] A recent clinical trial showed 2-DG can be tolerated at a dose of 63 mg/kg/day, however the observed cardiac side-effects (prolongation of the Q-T interval) at this dose and the fact that a majority of patients' (66%) cancer progressed casts doubt on the feasibility of this reagent for further clinical use.[4] However, it is not completely clear how 2-DG inhibits cell growth. The fact that glycolysis is inhibited by 2-DG, seems not to be sufficient to explain why 2-DG treated cells stop growing.[5] Because of its structural similarity to mannose, 2DG has the potential to inhibit N-glycosylation in mammalian cells and other systems, and as such induces ER stress and the Unfolded Protein Response (UPR) pathway.[6][7][8]
Clinicians have noted that 2-DG is metabolised in the pentose phosphate pathway in red blood cells at least, although the significance of this for other cell types and for cancer treatment in general is unclear.
Work on the ketogenic diet as a treatment for epilepsy have investigated the role of glycolysis in the disease. 2-Deoxyglucose has been proposed by Garriga-Canut et al. as a mimic for the ketogenic diet, and shows great promise as a new anti-epileptic drug.[9][10] The authors suggest that 2-DG works, in part, by increasing the expression of Brain-derived neurotrophic factor (BDNF), Nerve growth factor (NGF), Arc (protein) (ARC), and Basic fibroblast growth factor (FGF2).[11] Such uses are complicated by the fact that 2-deoxyglucose does have some toxicity.
A study found that by combining the sugar 2-deoxy-D-glucose (2-DG) with fenofibrate, a compound that has been safely used in humans for more than 40 years to lower cholesterol and triglycerides, an entire tumor could effectively be targeted without the use of toxic chemotherapy.[12][13]
2-DG has been used as a targeted optical imaging agent for fluorescent in vivo imaging.[14][15] In clinical medical imaging (PET scanning), fluorodeoxyglucose is used, where one of the 2-hydrogens of 2-deoxy-D-glucose is replaced with the positron-emitting isotope fluorine-18, which emits paired gamma rays, allowing distribution of the tracer to be imaged by external gamma camera(s). This is increasingly done in tandem with a CT function which is part of the same PET/CT machine, to allow better localization of small-volume tissue glucose-uptake differences.
Resistance to 2-DG has been reported in HeLa cells [16] and in yeast;[17][8] in the latter, it involves the detoxification of a metabolite derived from 2-DG (2DG-6-phosphate) by a phosphatase. Despite the existence of such a phosphatase in human (named HDHD1A) However it is unclear whether it contributes to the resistance of human cells to 2DG or affects FDG-based imaging.
References
- Merck Index, 11th Edition, 2886.
- Wick, AN; Drury, DR; Nakada, HI; Wolfe, JB (1957). "Localization of the primary metabolic block produced by 2-deoxyglucose" (PDF). J Biol Chem. 224 (2): 963–969. PMID 13405925.
- Pelicano, H; Martin, DS; Xu, RH; Huang, P (2006). "Glycolysis inhibition for anticancer treatment". Oncogene. 25 (34): 4633–4646. doi:10.1038/sj.onc.1209597. PMID 16892078.
- Raez, LE; Papadopoulos, K; Ricart, AD; Chiorean, EG; Dipaola, RS; Stein, MN; Rocha Lima, CM; Schlesselman, JJ; Tolba, K; Langmuir, VK; Kroll, S; Jung, DT; Kurtoglu, M; Rosenblatt, J; Lampidis, TJ (2013). "A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors". Cancer Chemother. Pharmacol. 71 (2): 523–30. doi:10.1007/s00280-012-2045-1. PMID 23228990. S2CID 2990078.
- Ralser, M.; Wamelink, M. M.; Struys, E. A.; Joppich, C.; Krobitsch, S.; Jakobs, C.; Lehrach, H. (2008). "A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth". Proceedings of the National Academy of Sciences. 105 (46): 17807–17811. Bibcode:2008PNAS..10517807R. doi:10.1073/pnas.0803090105. PMC 2584745. PMID 19004802.
- Kurtoglu, M.; Gao, N.; Shang, J.; Maher, J. C.; Lehrman, M. A.; Wangpaichitr, M.; Savaraj, N.; Lane, A. N.; Lampidis, T. J. (2007-11-07). "Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation". Molecular Cancer Therapeutics. 6 (11): 3049–3058. doi:10.1158/1535-7163.mct-07-0310. ISSN 1535-7163. PMID 18025288.
- Xi, Haibin; Kurtoglu, Metin; Liu, Huaping; Wangpaichitr, Medhi; You, Min; Liu, Xiongfei; Savaraj, Niramol; Lampidis, Theodore J. (2010-07-01). "2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion". Cancer Chemotherapy and Pharmacology. 67 (4): 899–910. doi:10.1007/s00280-010-1391-0. ISSN 0344-5704. PMC 3093301. PMID 20593179.
- Defenouillère, Quentin; Verraes, Agathe; Laussel, Clotilde; Friedrich, Anne; Schacherer, Joseph; Léon, Sébastien (2019-09-03). "The induction of HAD-like phosphatases by multiple signaling pathways confers resistance to the metabolic inhibitor 2-deoxyglucose". Science Signaling. 12 (597): eaaw8000. doi:10.1126/scisignal.aaw8000. ISSN 1945-0877. PMID 31481524. S2CID 201829818.
- Garriga-Canut, Mireia; Schoenike, Barry; Qazi, Romena; Bergendahl, Karen; Daley, Timothy J.; Pfender, Rebecca M.; Morrison, John F.; Ockuly, Jeffrey; Stafstrom, Carl; Sutula, Thomas; Roopra, Avtar (2006). "2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CTBP–dependent metabolic regulation of chromatin structure". Nature Neuroscience. 9 (11): 1382–1387. doi:10.1038/nn1791. PMID 17041593. S2CID 10175791.
- Garriga-Canut, M.; Schoenike, B.; Qazi, R.; Bergendahl, K.; Daley, T. J.; Pfender, R. M.; Morrison, J. F.; Ockuly, J.; Stafstrom, C.; Sutula, T.; Roopra, A. (2006). "2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP–dependent metabolic regulation of chromatin structure". Nature Neuroscience. 9 (11): 1382–1387. doi:10.1038/nn1791. PMID 17041593. S2CID 10175791.
- Jia Yao, Shuhua Chen, Zisu Mao, Enrique Cadenas, Roberta Diaz Brinton "2-Deoxy-D-Glucose Treatment Induces Ketogenesis, Sustains Mitochondrial Function, and Reduces Pathology in Female Mouse Model of Alzheimer's Disease", PLOS ONE
- Researchers develop novel, non-toxic approach to treating variety of cancers. ScienceDaily
- Liu, Huaping; Kurtoglu, Metin; León-Annicchiarico, Clara Lucia; Munoz-Pinedo, Cristina; Barredo, Julio; Leclerc, Guy; Merchan, Jaime; Liu, Xiongfei; Lampidis, Theodore J. (2016). "Combining 2-deoxy-D-glucose with fenofibrate leads to tumor cell death mediated by simultaneous induction of energy and ER stress". Oncotarget. 7 (24): 36461–36473. doi:10.18632/oncotarget.9263. PMC 5095013. PMID 27183907.
- Kovar, Joy L.; Volcheck, William; Sevick-Muraca, Eva; Simpson, Melanie A.; Olive, D. Michael (2009). "Characterization and performance of a near-infrared 2-deoxyglucose optical imaging agent for mouse cancer models". Analytical Biochemistry. 384 (2): 254–262. doi:10.1016/j.ab.2008.09.050. PMC 2720560. PMID 18938129.
- Cheng, Z., Levi, J., Xiong, Z., Gheysens, O., Keren, S., Chen, X., and Gambhir, S., Bioconjugate Chemistry, 17(3), (2006), 662-669
- Barban, Stanley (December 1962). "Induced resistance to 2-deoxy-d-glucose in cell cultures". Biochimica et Biophysica Acta. 65 (2): 376–377. doi:10.1016/0006-3002(62)91065-x. ISSN 0006-3002. PMID 13966473.
- Sanz, Pascual; Randez-Gil, Francisca; Prieto, José Antonio (September 1994). "Molecular characterization of a gene that confers 2-deoxyglucose resistance in yeast". Yeast. 10 (9): 1195–1202. doi:10.1002/yea.320100907. ISSN 0749-503X. PMID 7754708. S2CID 9497505.