2.2.2-Cryptand
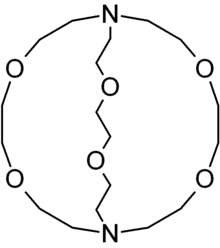
[2.2.2]-Cryptand is the organic compound with the formula N(CH2CH2OCH2CH2OCH2CH2)3N. This bicyclic molecule is the most studied member of the cryptand family of chelating agents.[2] It is a white solid. Many analogous compounds are known. Their high affinity for alkali metal cations illustrates the advantages of "preorganization", a concept within the area of supramolecular chemistry.
 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane | |||
| Other names
Cryptating agent 222[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | Crypt-222 | ||
| 620282 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.041.770 | ||
| EC Number |
| ||
| MeSH | Cryptating+agent+222 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C 18N 2H 36O 6 | |||
| Molar mass | 376.4882 g mol−1 | ||
| Melting point | 68 to 71 °C (154 to 160 °F; 341 to 344 K) | ||
| Hazards | |||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
| H315, H319, H335 | |||
| P261, P305+351+338 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
For the design and synthesis of [2.2.2]cryptand,[3] Jean-Marie Lehn shared the Nobel Prize in Chemistry. The compound was originally prepared starting with the diacylation of the diamine-diether:[4]
- [CH2OCH2CH2NH2]2 + [CH2OCH2COCl]2 → [CH2OCH2CH2NHC(O)CH2]2 + 2 HCl
The resulting macrocyclic diamide is reduced by lithium aluminium hydride. The resulting macrocyclic diamine tetraether reacts with a second equivalent of [CH2OCH2COCl]2 to produce the macrobicyclic diamide. This di(tertiary)amide is reduced to the diamine by diborane.
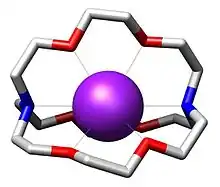
[2.2.2]Cryptand binds K+ as an octadentate N2O6 ligand. The resulting cation [K(cryptand)]+ is lipophilic.
References
- In the Nomenclature of Inorganic Chemistry (2005), IUPAC recommends the abbreviation "crypt-222".
- Kang, Sung Ok; Llinares, José M.; Day, Victor W.; Bowman-James, Kristin (2010). "Cryptand-like anion receptors". Chemical Society Reviews. 39 (10): 3980–4003. doi:10.1039/C0CS00083C. PMID 20820597.
- Dietrich, B.; Lehn, J.M.; Sauvage, J.P. (1969). "Les Cryptates". Tetrahedron Letters. 10 (34): 2889–2892. doi:10.1016/S0040-4039(01)88300-3.
- Dietrich, B.; Lehn, J.M.; Sauvage, J.P. (1969). "Diaza-polyoxa-macrocycles et macrobicycles". Tetrahedron Letters. 10 (34): 2885–2888. doi:10.1016/S0040-4039(01)88299-X.
- Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A. P. (2001). "Synthesis and properties of boranocarbonate: a convenient in situ CO source for the aqueous preparation of [99mTc(OH2)3(CO)3]+". J. Am. Chem. Soc. 121 (13): 3135–3136. doi:10.1021/ja003932b. PMID 11457025.