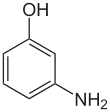
3-Aminophenol
3-Aminophenol is an organic compound with formula C6H4(NH2)(OH). It is an aromatic amine and aromatic alcohol. It is the meta isomer of 2-aminophenol and 4-aminophenol.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3-Aminophenol[2] | |||
| Other names
m-Aminophenol meta-Aminophenol 3-Hydroxyaniline m-Hydroxyaniline meta-Hydroxyaniline | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 636059 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.830 | ||
| EC Number |
| ||
| 2913 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2512 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H7NO | |||
| Molar mass | 109.128 g·mol−1 | ||
| Appearance | White orthorhombic crystals | ||
| Density | 1.195 g/cm3 | ||
| Melting point | 120 to 124 °C (248 to 255 °F; 393 to 397 K) | ||
| Boiling point | 164 °C (327 °F; 437 K) at 11 mmHg | ||
| Acidity (pKa) |
| ||
| Hazards | |||
| GHS pictograms |  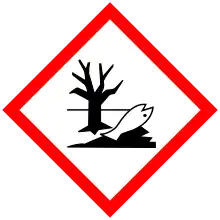 | ||
| GHS Signal word | Warning | ||
| H302, H332, H411 | |||
| P261, P264, P270, P271, P273, P301+312, P304+312, P304+340, P312, P330, P391, P501 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
3-Aminophenol can be prepared by caustic fusion of 3-aminobenzenesulfonic acid (i.e. heating with NaOH to 245 °C for 6 hours)[4] or from resorcinol via a substitution reaction with ammonium hydroxide.[5]
Uses
One of the most relevant applications of the substance is the synthesis of 3-(diethylamino)phenol, key intermediate for the preparation of several fluorescent dyes (e.g., rhodamine B). Other uses for the compound include hair dye colorants and stabilizers for chlorine-containing thermoplastics.[4]
References
- 3-Aminophenol at Sigma-Aldrich.
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature. The structure is substitutable at any position. Locants 2, 3, and 4 are recommended, not o, m, and p.
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–89. ISBN 978-1498754286.
- Mitchell, Stephen C.; Waring, Rosemary H. (2000). "Aminophenols". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_099.
- Harada, Haruhisa; Hiroshi, Maki; Sasaki, Shigeru (1986). "Method for the production of m-aminophenol EP0197633A1". Google Patents. Sumitomo Chemical Company, Limited. Retrieved 3 February 2015.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.