3-Hydroxybenzoyl-CoA
3-Hydroxybenzoyl-CoA is a molecule formed by condensing the thiol group of coenzyme A (CoA) with the carboxylic acid group of 3-hydroxybenzoic acid. Stable in acidic conditions, it is a tetraprotic acid due to the pyrophosphate and phosphate groups included. It derives from a benzoyl-CoA and a 3-hydroxybenzoic acid. In organisms such as plants, this can be formed using the 3-hydroxybenzoate—CoA ligase enzyme. This uses ATP, 3-hydroxybenzoate and CoA as substrates.[1]
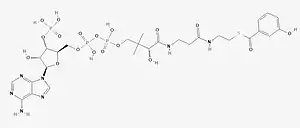 Chemical structure of 3-Hydroxybenzoyl-CoA | |
| Names | |
|---|---|
| IUPAC name
S-[2-[3-[[(2R)-4-[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethyl] 3-hydroxybenzenecarbothioate | |
| Other names
3-Hydroxybenzoyl-CoA
3-hydroxybenzoyl-coenzyme A m-hydroxybenzoyl-CoA m-hydroxybenzoyl-coenzyme A | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C28H40N7O18P3S | |
| Molar mass | 887.64 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It can be reduced to 3-hydroxycyclohexa-1,5-diene-1-carbonyl-CoA by reduced ferredoxin and adenosine triphosphate using the benzoyl-CoA reductase enzyme.[2] in this two hydrogen atoms are added to the benzene ring in a dearomatizing process.
References
- "BRENDA - Information on EC 6.2.1.37 - 3-hydroxybenzoate-CoA ligase". www.brenda-enzymes.org.
- "bcrA - Benzoyl-CoA reductase subunit A - Thauera aromatica - bcrA gene & protein". www.uniprot.org.