4-Methyl-3-thiosemicarbazide
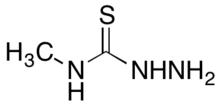
4-Methyl-3-thiosemicarbazide is an organosulfur compound with the formula of with the formula CH3NHC(S)NHNH2. It is a white, odorless solid. The compound is one of the many derivatives of thiosemicarbazide. It is a precursor to diverse organic compounds and metal complexes.
 | |
| Names | |
|---|---|
| Systematic IUPAC name
N-Methylhydrazinecarbothioamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.026.876 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H7N3S | |
| Molar mass | 105.16 g·mol−1 |
| Melting point | 135 to 138 °C (275 to 280 °F; 408 to 411 K)[1] |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H300 | |
| P264, P270, P301+310, P321, P330, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
14 mg/kg rat |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
4-Methyl-3-thiosemicarbazide is used as an intermediate compound in the synthesis of some types of herbicides, for example tebuthiuron.[2]
2_(BAKBEV).png.webp)
Structure of Ni(H2NNCSNHMe)2.[3]
Precautions and toxicity
4-Methyl-3-thiosemicarbazide can cause irritation of the eyes, respiratory tract, and skin. Swallowing the chemical may cause death.[1] It is classified as a "dangerous good for transport".[4]
References
- Material Safety Data Sheet, October 26, 2012, retrieved August 23, 2013
- Ralph Kenneth Achgill, Laurence Wesley Call (November 2, 1989), Improved synthesis of 4-methyl-3-thiosemicarbazide, retrieved August 24, 2013
- Burrows, Andrew D.; Harrington, Ross W.; Mahon, Mary F.; Teat, Simon J. (2002). "Structural manipulation through selective substitution of hydrogen bonding groups: The supramolecular structures of bis(thiosemicarbazidato)nickel complexes". CrystEngComm. 4 (89): 539. doi:10.1039/b208284e.
- 4-Methyl-3-thiosemicarbazide (CAS 6610-29-3), 2007, retrieved August 24, 2013
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.