A3 coupling reaction
The A3 coupling (also known as A3 coupling reaction or the aldehyde-alkyne-amine reaction), termed by Prof. Chao-Jun Li of McGill University, is a type of multicomponent reaction involving an aldehyde, an alkyne and an amine which react to give a propargyl-amine.[1][2][3][4][5]
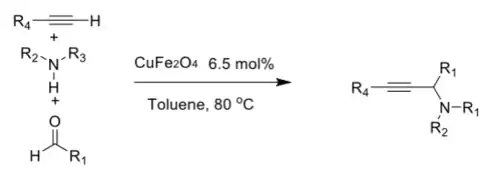
The reaction proceeds via direct dehydrative condensation[3] and requires a metal catalyst, typically based on ruthenium/copper, gold or silver.[3] Chiral catalyst can be used to give an enantioselective reaction, yielding a chiral amine. The solvent can be water.[3] In the catalytic cycle the metal activates the alkyne to a metal acetylide, the amine and aldehyde combine to form an imine which then reacts with the acetylide in a nucleophilic addition.[3] The reaction type was independently reported by three research groups in 2001 -2002;[6][7][8] one report on a similar reaction dates back to 1953.[9][10]
If the amine substituents have an alpha hydrogen present and provided a suitable zinc or copper catalyst is used, the A3 coupling product may undergo a further internal hydride transfer and fragmentation to give an allene in a Crabbé reaction.
Decarboxylative A3 reaction
One variation is called the decarboxylative A3 coupling.[11] In this reaction the amine is replaced by an amino acid. The imine can isomerise and the alkyne group is placed at the other available nitrogen alpha position.[11][12][13] This reaction requires a copper catalyst. The redox A3 coupling has the same product outcome but the reactants are again an aldehyde, an amine and an alkyne as in the regular A3 coupling.[11][14][15][16]
References
- W.-J. Yoo, L. Zhao, C.-J. Li, Aldrichimica Acta 2011, 44, 43–51. The A3-coupling (aldehyde-alkyne-amine) reaction: a versatile method for the preparation of propargylamines
- A walk around the A3-coupling Vsevolod A. Peshkov , Olga P. Pereshivkoa and Erik V. Van der Eycken Chem. Soc. Rev., 2012,41, 3790-3807 doi:10.1039/C2CS15356D
- Name Reactions A Collection of Detailed Mechanisms and Synthetic Applications 2009 Li, Jie Jack
- The Development of A3-Coupling (Aldehyde-Alkyne-Amine) and AA3-Coupling (Asymmetric Aldehyde-Alkyne-Amine) Chunmei Wei, Zigang Li, Chao-Jun Li Synlett 2004(9): 1472-1483 doi:10.1055/s-2004-829531
- Rokade, Balaji V.; Barker, James; Guiry, Patrick J. (2019). "Development of and recent advances in asymmetric A3 coupling". Chemical Society Reviews. doi:10.1039/C9CS00253G.
- Sakaguchi, S., Kubo, T. and Ishii, Y. (2001), A Three-Component Coupling Reaction of Aldehydes, Amines, and Alkynes. Angew. Chem. Int. Ed., 40: 2534–2536. doi: 10.1002/1521-3773(20010702)40:13<2534::AID-ANIE2534>3.0.CO;2-2
- Direct Addition of TMS-acetylene to Aldimines Catalyzed by a Simple, Commercially Available Ir(I) Complex Christian Fischer and Erick M. Carreira Organic Letters 2001 3 (26), 4319-4321 doi: 10.1021/ol017022q
- Highly efficient Grignard-type imine additions via C–H activation in water and under solvent-free conditions Chao-Jun Li and Chunmei Wei Chem. Commun., 2002, 268-269 doi:10.1039/B108851N
- Guermont, J. P. Bull. Soc. Chim. Fr. 1953, 386.
- Recent advances on diversity oriented heterocycle synthesis via multicomponent tandem reactions based on A3 coupling Yunyun Liu ARKIVOC 2014 (i) 1-20
- The redox-A3 reaction Daniel Seidel Org. Chem. Front., 2014, 1, 426 doi:10.1039/c4qo00022f
- Aldehyde- and Ketone-Induced Tandem Decarboxylation-Coupling (Csp3−Csp) of Natural α-Amino Acids and Alkynes Hai-Peng Bi, Qingfeng Teng, Min Guan, Wen-Wen Chen, Yong-Min Liang, Xiaojun Yao, and Chao-Jun Li The Journal of Organic Chemistry 2010 75 (3), 783-788 doi:10.1021/jo902319h
- Nontraditional Reactions of Azomethine Ylides: Decarboxylative Three-Component Couplings of α-Amino Acids Chen Zhang and Daniel Seidel Journal of the American Chemical Society 2010 132 (6), 1798-1799 doi:10.1021/ja910719x
- Das, D., Sun, A. X. and Seidel, D. (2013), Redox-Neutral Copper(II) Carboxylate Catalyzed α-Alkynylation of Amines. Angew. Chem. Int. Ed., 52: 3765–3769. doi:10.1002/anie.201300021
- CuI-Catalyzed C1-Alkynylation of Tetrahydroisoquinolines (THIQs) by A3 Reaction with Tunable Iminium Ions Qin-Heng Zheng, Wei Meng, Guo-Jie Jiang, and Zhi-Xiang Yu Organic Letters 2013 15 (23), 5928-5931 doi:10.1021/ol402517e
- Lin, W., Cao, T., Fan, W., Han, Y., Kuang, J., Luo, H., Miao, B., Tang, X., Yu, Q., Yuan, W., Zhang, J., Zhu, C. and Ma, S. (2014), Enantioselective Double Manipulation of Tetrahydroisoquinolines with Terminal Alkynes and Aldehydes under Copper(I) Catalysis. Angew. Chem. Int. Ed., 53: 277–281. doi:10.1002/anie.201308699