Acetyl nitrate
Acetyl nitrate is the organic compound with the formula CH3C(O)ONO2. It is classified as the mixed anhydride of nitric and acetic acids. It is a colorless explosive liquid that fumes in moist air.
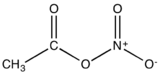 | |
| Names | |
|---|---|
| Preferred IUPAC name
Acetic nitric anhydride | |
| Other names
Acetyl nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3NO4 | |
| Molar mass | 105.05 |
| Appearance | colorless liquid |
| Density | 1.24 g/cm3 (15 °C) |
| Boiling point | 22 °C at 70 Torr [1] |
| Hazards | |
| Main hazards | explosion |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
It is prepared from acetic anhydride and dinitrogen pentoxide or with nitric acid:
- (CH3CO)2O + HNO3 → CH3C(O)ONO2 + CH3CO2H
It hydrolyzes in air to acetic and nitric acid, the reverse of the above reaction. Alternatively, nitric acid adds to ketene.
It is used for some nitrations and nitrolysis reactions.[2] It acetylates amines, akin to the behavior of acetyl chloride:
- 2 RNH2 + CH3C(O)ONO2 → [RNH3]NO3 + CH3C(O)NHR
References
- A. Pictet, E. Khotinsky: Über Acetylnitrat. in Chem. Ber. 40, 1907, S. 1163–1166, doi:10.1002/cber.190704001172.
- Louw, Robert "Acetyl nitrate" e-EROS Encyclopedia of Reagents for Organic Synthesis 2001, 1-2. doi: 10.1002/047084289X.ra032
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.