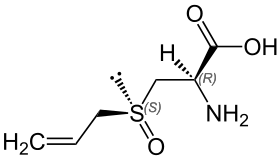
Alliin
Alliin /ˈæli.ɪn/ is a sulfoxide that is a natural constituent of fresh garlic.[1] It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2R)-2-amino-3-[(S)-prop-2-enylsulfinyl]propanoic acid | |
| Other names
3-(2-Propenylsulfinyl)alanine (S)-3-(2-Propenylsulfinyl)-L-alanine 3-((S)-Allylsulfinyl)-L-alanine S-Allyl-L-cysteine sulfoxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.291 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H11NO3S | |
| Molar mass | 177.22 g/mol |
| Appearance | White to off white crystalline powder |
| Melting point | 163–165 °C (325–329 °F) |
| Soluble | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Garlic has been used since antiquity as a therapeutic remedy for certain conditions now associated with oxygen toxicity, and, when this was investigated, garlic did indeed show strong antioxidant and hydroxyl radical-scavenging properties, it is presumed owing to the alliin contained within.[2] Alliin has also been found to affect immune responses in blood.[3]
Alliin was the first natural product found to have both carbon- and sulfur-centered stereochemistry.[4]
Chemical synthesis
The first reported synthesis, by Stoll and Seebeck in 1951,[5] begins the alkylation of L-cysteine with allyl bromide to form deoxyalliin. Oxidation of this sulfide with hydrogen peroxide gives both diastereomers of L-alliin, differing in the orientation of the oxygen atom on the sulfur stereocenter.
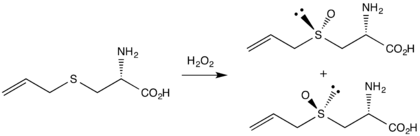
A newer route, reported by Koch and Keusgen in 1998,[6] allows stereospecific oxidation using conditions similar to the Sharpless asymmetric epoxidation. The chiral catalyst is produced from diethyl tartrate and titanium isopropoxide.
References
- Iberl, B; et al. (1990). "Quantitative Determination of Allicin and Alliin from Garlic by HPLC". Planta Med. 56 (3): 320–326. doi:10.1055/s-2006-960969. PMID 17221429.
- Kourounakis, PN; Rekka, EA (November 1991). "Effect on active oxygen species of alliin and Allium sativum (garlic) powder". Res Commun Chem Pathol Pharmacol. 74 (2): 249–252. PMID 1667340.
- Salman, H; et al. (September 1999). "Effect of a garlic derivative (alliin) on peripheral blood cell immune responses". Int J Immunopharmacol. 21 (9): 589–597. doi:10.1016/S0192-0561(99)00038-7. PMID 10501628.
- Eric Block (2009). Garlic and other alliums: the lore and the science. Royal Society of Chemistry. pp. 100–106.
- Stoll , Seeback, Arthur, Ewald (1951). "Chemical investigations on alliin, the specific principle of garlic". Advanced Enzymology. 11: 377–400.
- Koch, Keusgen (1998). "Diastereoselective synthesis of alliin by an asymmetric sulfur oxidation". Pharmazie. 53 (668–671).
