Amabiline
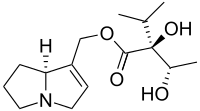
Amabiline is a pyrrolizidine alkaloid first isolated in 1967 from Cynoglossum amabile.[1] It is also found in the seeds and flowers of borage (Borago officinalis)[2] and in borage seed oil.[3][4]
 | |
| Names | |
|---|---|
| IUPAC name
[(8S)-5,6,7,8-Tetrahydro-3H-pyrrolizin-1-yl]methyl (2S)-2-hydroxy-2-[(1S)-1-hydroxyethyl]-3-methylbutanoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H25NO4 | |
| Molar mass | 283.368 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemically, it is the ester derived from viridifloric acid and supinidine.[5]
Amabiline is hepatoxic and may contribute to the potential liver damage caused by consumption of borage and its seed oil.[6]
References
- Culvenor, CCJ; Smith, LW (1967). "The alkaloids of Cynoglossum australe R.Br. And C. Amabile Stapf & Drummond". Australian Journal of Chemistry. 20 (11): 2499. doi:10.1071/CH9672499.
- Dodson, Craig D.; Stermitz, Frank R. (1986). "Pyrrolizidine Alkaloids from Borage (Borago officinalis) Seeds and Flowers". Journal of Natural Products. 49 (4): 727–728. doi:10.1021/np50046a045.
- Parvais, O.; Vander Stricht, B.; Vanhaelen-Fastre, R.; Vanhaelen, M. (1994). "TLC detection of pyrrolizidine alkaloids in oil extracted from the seeds of Borago officinalis". Journal of Planar Chromatography--Modern TLC. 7 (1): 80–82.
- Wretensjoe, Inger; Karlberg, Bo. (2003). "Pyrrolizidine alkaloid content in crude and processed borage oil from different processing stages". Journal of the American Oil Chemists' Society. 80 (10): 963–970. doi:10.1007/s11746-003-0804-z.
- The Alkaloids: Chemistry and Physiology. Academic Press. 1970. pp. 302–303.
- "Borage". Memorial Sloan Kettering Cancer Center.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.