Anthranilate synthase
In enzymology, an anthranilate synthase (EC 4.1.3.27) is an enzyme that catalyzes the chemical reaction
- chorismate + L-glutamine anthranilate + pyruvate + L-glutamate 2
Anthranilate Synthase 1i1q
 One of many anthranilate synthase structures. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.1.3.27 | ||||||||
| CAS number | 9031-59-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Thus, the two substrates of this enzyme are chorismate and L-glutamine, whereas its 3 products are anthranilate, pyruvate, and L-glutamate.
Reaction
In enzymology, an anthranilate synthase (EC 4.1.3.27) is an enzyme that catalyzes the chemical reaction
- chorismate + L-glutamine anthranilate + pyruvate + L-glutamate
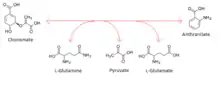
Thus, the two substrates of this enzyme are chorismate and L-glutamine, whereas its 3 products are anthranilate, pyruvate, and L-glutamate.
Structure and assembly
The complex is made up of α and β subunits. Gel filtration experiments reveal that the complex occurs as an α2β2 tetramer under native conditions, and as an αβ dimer under high salt concentrations.[1] The αβ dimers interact through the α subunits to form the complex.
Nomenclature
This enzyme belongs to the family of lyases, to be specific the oxo-acid-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is chorismate pyruvate-lyase (amino-accepting; anthranilate-forming). Other names in common use include anthranilate synthetase, chorismate lyase, and chorismate pyruvate-lyase (amino-accepting). This enzyme participates in phenylalanine, tyrosine and tryptophan biosynthesis and two-component system - general.
Structural studies
As of late 2007, 5 structures have been solved for this class of enzymes, with PDB accession codes 1I1Q, 1I7Q, 1I7S, 1QDL, and 2I6Y.
References
- Poulsen, C; Bongaerts, RJ; Verpoorte, R (March 1993). "urification and characterization of anthranilate synthase from Catharanthus roseus". European Journal of Biochemistry. 212 (2): 431–40. doi:10.1111/j.1432-1033.1993.tb17679.x. PMID 8444181.
- Baker TI, Crawford IP (1966). "Anthranilate synthetase. Partial purification and some kinetic studies on the enzyme from Escherichia coli". J. Biol. Chem. 241 (23): 5577–84. PMID 5333199.
- Creighton TE, Yanofsky C (1970). "Chorismate to tryptophan (Escherichia coli) - Anthranilate synthetase, PR transferase, PRA isomerase, InGP synthetase, tryptophan synthetase". Methods Enzymol. 17A: 365–380. doi:10.1016/0076-6879(71)17215-1.
- Kung CC, Huang WN, Huang YC, Yeh KC (2006). "Proteomic survey of copper-binding proteins in Arabidopsis roots by immobilized metal affinity chromatography and mass spectrometry". Proteomics. 6 (9): 2746–58. doi:10.1002/pmic.200500108. PMID 16526091. S2CID 25896917.
- Ito J, Yanofsky C (1969). "Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: Comparative studies on the complex and the subunits". J. Bacteriol. 97 (2): 734–42. doi:10.1128/JB.97.2.734-742.1969. PMC 249753. PMID 4886290.
- Zalkin H, Kling D (1968). "Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium". Biochemistry. 7 (10): 3566–73. doi:10.1021/bi00850a034. PMID 4878701.