Arterial input function
Arterial input function (AIF), also known as a plasma input function, refers to the concentration of tracer in blood-plasma in an artery measured over time. The oldest record on PubMed shows that AIF was used by Harvey et al.[1] in 1962 to measure the exchange of materials between red blood cells and blood plasma, and by other researchers in 1983 for positron emission tomography (PET) studies.[2][3] Nowadays, kinetic analysis is performed in various medical imaging techniques, which requires an AIF as one of the inputs to the mathematical model, for example, in dynamic PET imaging,[4] or perfusion CT,[5] or dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI).[6][7]
How is AIF obtained
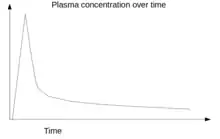
AIF can be obtained in several different ways, for example, using the invasive method of continuous arterial sampling using an online blood monitor,[8] using the invasive method of arterial blood samples obtained at discrete time points post-injection,[4] using a minimally invasive method using a population-based AIF where an input function in a subject is estimated partly from the prior information obtained from a previous population and partly from the blood information from the subject itself obtained at the time of scanning,[9] or using an image-derived arterial input function (IDAIF) obtained by placing a region of interest (ROI) over an artery and calibrating the resulting curves against venous blood samples obtained during the later phases (30 to 60 minutes) of the dynamic scan[10] when venous and arterial tracer concentrations become equal.[4]
A dynamic scan is a scan where two dimensional (2D) or three dimensional (3D) images are acquired again and again over a time-period forming a time-series of 2D/3D image datasets. For example, a dynamic PET scan acquired over a period of one hour contains the first few short image frames acquired for 5 seconds duration to capture the fast dynamics of the tracer immediately after a tracer-injection and later frames acquired for 30 seconds. Each data-point in the AIF curve represents a measurement of tracer-concentration from an artery obtained from each of these image time-frame acquired over time, with external corrections applied to it.
These four methods are briefly described as follows:
Continuous arterial sampling
Continuous arterial blood sampling is invasive, painful, and uncomfortable for the patients. Continuous arterial sampling was obtained in postmenopausal women imaged using [18F]NaF for bone studies.[11]
Discrete arterial sampling
Discrete arterial blood sampling is invasive, painful, and uncomfortable for the patients. Cook et al. measured discrete blood samples and compared them to continuous arterial sampling in postmenopausal women imaged using [18F]NaF for bone studies.[11] Another study in head and neck cancer patients imaged using [18F]FLT PET, and numerous other studies, obtained discrete arterial samples for the estimation of arterial input function.[12]
The approach of obtaining discrete arterial sampling was based on the observation that the bolus peak occurs with 5 minutes after injection, and that the latter part of the curve, in most cases, represent a single or bi-exponential curve. It implied that continuous arterial sampling was not necessary, and the discrete arterial blood samples were enough to obtain the continuous curves using an exponential model fit.
Population-based method
A population-based input function generally relies on the dataset previously obtained by other researchers in a specific set of populations, and average values are used. The methods generally provide better results if a large number of datasets is used and based on the assumption that the input function in a new patient in this sub-group of the population will be insignificantly different from the population average values. In a neuroinflammation study, the author using a population-based input function in healthy volunteers and liver-transplanted patients imaged using [18F]GE-180 PET.[13] In another study, healthy controls and patients with Parkinson's and Alzheimer's disease were imaged using [18F]FEPPA PET.[14] Zanotti-Fregonara et al.[15] thoroughly reviewed the literature on the arterial input function used for brain PET imaging and suggested the possibility of population-based arterial input functions as a potential alternative to invasive arterial sampling.
However, Blake et al.[16] derived a semi-population based method from healthy postmenopausal women imaged using [18F]NaF for bone studies[16] based on the observation that the later part of the arterial input function can be constructed from the venous blood samples, as the venous and arterial blood concentration of tracer is equal 30 minutes after the injection. They derived the peak of the curve from a previous study that obtained continuous arterial sampling, and the later part of the curve from the venous blood samples of the individual patient in whom an AIF is to be estimated. When combined, a semi-population based arterial input function is obtained as a result.
Image-derived method
An image-derived arterial input function (IDAIF) obtained by measuring the tracer counts over the aorta,[4] carodit artery,[17] or radial artery[8] offers an alternative to invasive arterial blood sampling. An IDAIF at the aorta can be determined by measuring the tracer counts over the left ventricle, ascending aorta, and abdominal aorta and this has been previously validated by various researchers.[10][4]
The arterial time-activity curve (TAC) from the image data requires corrections for metabolites formed over time, differences between whole blood and plasma activity, which are not constant over time, correction for partial volume errors (PVE) due to the small size of the ROI, spill-over errors due to activity from neighbouring tissues outside the ROI,[18] error due to patient movement, and noise introduced due to the limited number of counts acquired in each image time frame because of the short time frames. These errors are corrected using late venous blood samples,[4][10] and the resulting curve is called an arterial input function (AIF). There are numerous methods tried by researchers over the years.[19][20][21][22]
See also
References
- HARVEY, RB (1962). "Renal extraction of para-aminohippurate and creatinine measured by continuous in vivo sampling of arterial and renal-vein blood". Ann N Y Acad Sci. 102 (1): 46–54. Bibcode:1962NYASA.102...46H. doi:10.1111/j.1749-6632.1962.tb13624.x. PMID 13960801.
- Herscovitch, P (1983). "Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis". J Nucl Med. 24 (9): 782–9. PMID 6604139.
- Henze, E.; Huang, S. C.; Ratib, O.; Hoffman, E.; Phelps, M. E.; Schelbert, H. R. (1983). "Measurements of regional tissue and blood-pool radiotracer concentrations from serial tomographic images of the heart". J Nucl Med. 24 (11): 987–96. PMID 6605418.
- Cook, Gary J. R.; Lodge, Martin A.; Marsden, Paul K.; Dynes, Angela; Fogelman, Ignac (1999). "Non-invasive assessment of skeletal kinetics using fluorine-18 fluoride positron emission tomography: evaluation of image and population-derived arterial input functions". European Journal of Nuclear Medicine and Molecular Imaging. 26 (11): 1424–1429. doi:10.1007/s002590050474. ISSN 1619-7070. PMID 10552083. S2CID 22595140.
- Lui, Y.W.; Tang, E.R.; Allmendinger, A.M.; Spektor, V. (2010). "Evaluation of CT Perfusion in the Setting of Cerebral Ischemia: Patterns and Pitfalls". American Journal of Neuroradiology. 31 (9): 1552–1563. doi:10.3174/ajnr.a2026. ISSN 0195-6108. PMID 20190208.
- Schabel, Matthias C. (2012-01-31). "A unified impulse response model for DCE-MRI". Magnetic Resonance in Medicine. 68 (5): 1632–1646. doi:10.1002/mrm.24162. ISSN 0740-3194. PMID 22294448.
- Tanuj Puri, Sarah Wiscombe, Sally Marshall, John Simpson, Josephine Naish, Pete Thelwall. Changes in pulmonary vascular properties in a human model of acute lung injury measured using DCE-MRI, In 20th Annual Scientific Meeting of the British Chapter of International Society for Magnetic Resonance in Medicine (ISMRM), Edinburgh, UK, September 2014
- Marques, Tiago Reis; Ashok, Abhishekh H.; Angelescu, Ilinca; Borgan, Faith; Myers, Jim; Lingford-Hughes, Anne; Nutt, David J.; Veronese, Mattia; Turkheimer, Federico E.; Howes, Oliver D. (2020-04-15). "GABA-A receptor differences in schizophrenia: a positron emission tomography study using [11C]Ro154513". Molecular Psychiatry. doi:10.1038/s41380-020-0711-y. ISSN 1359-4184. PMID 32296127.
- Blake, Glen Mervyn; Siddique, Musib; Puri, Tanuj; Frost, Michelle Lorraine; Moore, Amelia Elizabeth; Cook, Gary James R.; Fogelman, Ignac (2012). "A semipopulation input function for quantifying static and dynamic 18F-fluoride PET scans". Nuclear Medicine Communications. 33 (8): 881–888. doi:10.1097/MNM.0b013e3283550275. ISSN 0143-3636. PMID 22617486. S2CID 42973690.
- Puri, Tanuj; Blake, Glen M.; Siddique, Musib; Frost, Michelle L.; Cook, Gary J.R.; Marsden, Paul K.; Fogelman, Ignac; Curran, Kathleen M. (2011). "Validation of new image-derived arterial input functions at the aorta using 18F-fluoride positron emission tomography". Nuclear Medicine Communications. 32 (6): 486–495. doi:10.1097/MNM.0b013e3283452918. ISSN 0143-3636. PMID 21386733. S2CID 32105830.
- Cook, Gary J. R.; Lodge, Martin A.; Blake, Glen M.; Marsden, Paul K.; Fogelman, Ignac (2010-02-18). "Differences in Skeletal Kinetics Between Vertebral and Humeral Bone Measured by 18F-Fluoride Positron Emission Tomography in Postmenopausal Women". Journal of Bone and Mineral Research. 15 (4): 763–769. doi:10.1359/jbmr.2000.15.4.763. ISSN 0884-0431. PMID 10780868.
- Hackett, Sara L; Liu, Dan; Chalkidou, Anastasia; Marsden, Paul; Landau, David; Fenwick, John D (2013). "Estimation of input functions from dynamic [18F]FLT PET studies of the head and neck with correction for partial volume effects". EJNMMI Research. 3 (1): 84. doi:10.1186/2191-219X-3-84. ISSN 2191-219X. PMC 4109699. PMID 24369816.
- Buchert, Ralph; Dirks, Meike; Schütze, Christian; Wilke, Florian; Mamach, Martin; Wirries, Ann-Katrin; Pflugrad, Henning; Hamann, Linda; Langer, Laura B.N.; Wetzel, Christian; Lukacevic, Mario (2020-04-23). "Reliable quantification of 18F-GE-180 PET neuroinflammation studies using an individually scaled population-based input function or late tissue-to-blood ratio". European Journal of Nuclear Medicine and Molecular Imaging. doi:10.1007/s00259-020-04810-1. ISSN 1619-7070. PMID 32322915.
- Mabrouk, Rostom; Strafella, Antonio P.; Knezevic, Dunja; Ghadery, Christine; Mizrahi, Romina; Gharehgazlou, Avideh; Koshimori, Yuko; Houle, Sylvain; Rusjan, Pablo (2017-05-17). Garg, Pradeep (ed.). "Feasibility study of TSPO quantification with [18F]FEPPA using population-based input function". PLOS ONE. 12 (5): e0177785. Bibcode:2017PLoSO..1277785M. doi:10.1371/journal.pone.0177785. ISSN 1932-6203. PMID 28545084.
- Zanotti-Fregonara, Paolo; Chen, Kewei; Liow, Jeih-San; Fujita, Masahiro; Innis, Robert B (2011-08-03). "Image-Derived Input Function for Brain PET Studies: Many Challenges and Few Opportunities". Journal of Cerebral Blood Flow & Metabolism. 31 (10): 1986–1998. doi:10.1038/jcbfm.2011.107. ISSN 0271-678X. PMC 3208145. PMID 21811289.
- Blake, Glen Mervyn; Siddique, Musib; Puri, Tanuj; Frost, Michelle Lorraine; Moore, Amelia Elizabeth; Cook, Gary James R.; Fogelman, Ignac (August 2012). "A semipopulation input function for quantifying static and dynamic 18F-fluoride PET scans". Nuclear Medicine Communications. 33 (8): 881–888. doi:10.1097/MNM.0b013e3283550275. ISSN 0143-3636. PMID 22617486. S2CID 42973690.
- Sari, Hasan; Erlandsson, Kjell; Law, Ian; Larsson, Henrik BW; Ourselin, Sebastien; Arridge, Simon; Atkinson, David; Hutton, Brian F (2017). "Estimation of an image derived input function with MR-defined carotid arteries in FDG-PET human studies using a novel partial volume correction method". Journal of Cerebral Blood Flow & Metabolism. 37 (4): 1398–1409. doi:10.1177/0271678X16656197. ISSN 0271-678X. PMC 5453460. PMID 27342321.
- Nuyts, J (1996). "Three-Dimensional Correction for Spillover and Recovery of Myocardial PET Images". Journal of Nuclear Medicine. 37 (5): 767–74. PMID 8965143.
- van der Weerdt, Arno P.; Boellaard, Ronald; Visser, Frans C.; Lammertsma, Adriaan A. (2007-02-27). "Accuracy of 3D acquisition mode for myocardial FDG PET studies using a BGO-based scanner". European Journal of Nuclear Medicine and Molecular Imaging. 34 (9): 1439–1446. doi:10.1007/s00259-007-0367-8. ISSN 1619-7070. PMID 17333179.
- Puri, Tanuj; Blake, Glen M.; Siddique, Musib; Frost, Michelle L.; Cook, Gary J.R.; Marsden, Paul K.; Fogelman, Ignac; Curran, Kathleen M. (June 2011). "Validation of new image-derived arterial input functions at the aorta using 18F-fluoride positron emission tomography". Nuclear Medicine Communications. 32 (6): 486–495. doi:10.1097/mnm.0b013e3283452918. ISSN 0143-3636. PMID 21386733.
- Lee, Jae-Hoon; Liow, Jeih-San; Paul, Soumen; Morse, Cheryl L.; Haskali, Mohammad B.; Manly, Lester; Shcherbinin, Sergey; Ruble, J. Craig; Kant, Nancy; Collins, Emily C.; Nuthall, Hugh N. (2020-03-14). "PET quantification of brain O-GlcNAcase with [18F]LSN3316612 in healthy human volunteers". EJNMMI Research. 10 (1): 20. doi:10.1186/s13550-020-0616-4. ISSN 2191-219X. PMC 7072082. PMID 32172476.
- Ringheim, Anna; Campos Neto, Guilherme de Carvalho; Anazodo, Udunna; Cui, Lumeng; da Cunha, Marcelo Livorsi; Vitor, Taise; Martins, Karine Minaif; Miranda, Ana Cláudia Camargo; de Barboza, Marycel Figols; Fuscaldi, Leonardo Lima; Lemos, Gustavo Caserta (2020-02-24). "Kinetic modeling of 68Ga-PSMA-11 and validation of simplified methods for quantification in primary prostate cancer patients". EJNMMI Research. 10 (1). doi:10.1186/s13550-020-0594-6. ISSN 2191-219X. PMID 32140850.