Atomic spacing
Atomic spacing refers to the distance between the nuclei of atoms in a material. This space is extremely large compared to the size of the atomic nucleus, and is related to the chemical bonds which bind atoms together.[1] In solid materials, the atomic spacing is described by the bond lengths of its atoms. In ordered solids, the atomic spacing between two bonded atoms is generally around a few ångströms (Å), which is on the order of 10−10 meters. However, in very low density gasses (for example, in outer space) the average distance between atoms can be as large as a meter. In this case, the atomic spacing isn't referring to bond length.
The atomic spacing of crystalline structures is usually determined by passing an electromagnetic wave of known frequency through the material, and using the laws of diffraction to determine its atomic spacing. The atomic spacing of amorphous materials (such as glass) varies substantially between different pairs of atoms, therefore diffraction cannot be used to accurately determine atomic spacing. In this case, the average bond length is a common way of expressing the distance between its atoms.
Example
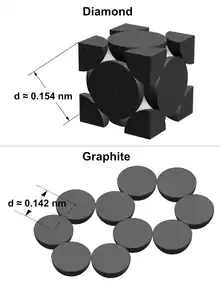
Bond length can be determined between different elements in molecules by using the atomic radii of the atom. Carbon bonds with itself to form two covalent network solids.[2] Diamond's C-C bond has a distance of Sqrt[3]a/4 ≈ 0.154 nm away from each carbon since adiamond ≈ 0.357 nm, while graphite's C-C bond has a distance of a/Sqrt[3] ≈ 0.142 nm away from each carbon since agraphite ≈ 0.246 nm. Although both bonds are between the same pair of elements they can still have different bond length.[3]
References
- Kittel, Charles (2004-11-11). Introduction to Solid State Physics (8th ed.). Wiley. ISBN 047141526X.
- Rossi, Miriam. "How can graphite and diamond be so different if they are both composed of pure carbon?". Scientific American. Scientific American. Retrieved October 9, 2007.
- Brown; Lemay; Bursten (1997). Chemistry the Central Science. Upper Saddle River, Nj: Simon and Schuster. pp. 412–413.