BSTFA
N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) is a chemical compound that is used to derivatise labile groups such as hydroxyl on other chemicals, with the more stable trimethylsilyl group, which protects the labile group and allows the compound to be used for analytical purposes or as a chemical reagent for synthesis of more complex molecules.[1] Siloxanes are usually more volatile than the corresponding hydroxyl compounds, and thus can be analyzed with gas chromatography better than the parent compound.
| |||
| Names | |||
|---|---|---|---|
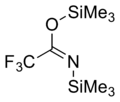
| IUPAC name
trimethylsilyl 2,2,2-trifluoro-N-trimethylsilylethanimidate | |||
| Other names
BSTFA, N,O-Bis(trimethylsilyl)trifluoroacetamide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.042.807 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C8H18F3NOSi2 | |||
| Molar mass | 257.403 g·mol−1 | ||
| Appearance | colourless | ||
| Density | 0.96 | ||
| Melting point | −10 °C (14 °F; 263 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.