Bacterial circadian rhythm
Bacterial circadian rhythms, like other circadian rhythms, are endogenous "biological clocks" that have the following three characteristics: (a) in constant conditions (i.e. constant temperature and either constant light {LL} or constant darkness {DD}) they oscillate with a period that is close to, but not exactly, 24 hours in duration, (b) this "free-running" rhythm is temperature compensated, and (c) the rhythm will entrain to an appropriate environmental cycle.
Until the mid-1980s, it was thought that only eukaryotic cells had circadian rhythms. It is now known that cyanobacteria (a phylum of photosynthetic eubacteria) have well-documented circadian rhythms that meet all the criteria of bona fide circadian rhythms. In these bacteria, three key proteins whose structures have been determined can form a molecular clockwork that orchestrates global gene expression. This system enhances the fitness of cyanobacteria in rhythmic environments.
History: are prokaryotes capable of circadian rhythmicity?
Before the mid-1980s, it was believed that only eukaryotes had circadian systems.[1] The conclusion that only eukaryotes have circadian oscillators seemed reasonable, because it was assumed that an endogenous timekeeper with a period close to 24 hours would not be useful to prokaryotic organisms that often divide more rapidly than once every 24 hours. The assumption might be stated as, "why have a timer for a cycle that is longer than your lifetime?" While intuitive, the conclusion was flawed. It was based on the assumption that a bacterial cell is equivalent to a sexually reproducing multicellular organism. However, a bacterial culture is more like a mass of protoplasm that grows larger and larger and incidentally subdivides. From this perspective, it is reasonable that a 24-hour temporal program could be adaptive to a rapidly dividing protoplasm if the fitness of that protoplasm changes as a function of daily alterations in the environment (light intensity, temperature, etc.).
In 1985–6, several research groups discovered that cyanobacteria display daily rhythms of nitrogen fixation in both light/dark (LD) cycles and in constant light. The group of Huang and co-workers was the first to recognize clearly that the cyanobacterium Synechococcus sp. RF-1 was exhibiting circadian rhythms, and in a series of publications beginning in 1986 demonstrated all three of the salient characteristics of circadian rhythms described above in the same organism, the unicellular freshwater Synechococcus sp. RF-1.[2][3] Another ground-breaking study was that of Sweeney and Borgese,[4] who were the first to demonstrate temperature compensation of a daily rhythm in the marine cyanobacterium, Synechococcus WH7803.
Inspired by the research of the aforementioned pioneers, the cyanobacterium Synechococcus elongatus was genetically transformed with a luciferase reporter that allowed rhythmic gene expression to be assayed non-invasively as rhythmically "glowing" cells.[5][6] This system allowed an exquisitely precise circadian rhythm of luminescence to be measured from cell populations[5] and even from single cyanobacterial cells.[7] The luminescence rhythms expressed by these transformed S. elongatus fulfilled all three key criteria of circadian rhythms: persistence of a 24-hour oscillation in constant conditions, temperature compensation, and entrainment. Thus, the work with various Synechococcus species firmly established that prokaryotic bacteria are capable of circadian rhythmicity, displacing the prior "no circadian clocks in prokaryotes" dogma. Nevertheless, persuasive evidence for circadian programs in bacteria other than the cyanobacteria is still lacking.
Relationship to cell division
Despite predictions that circadian clocks would not be expressed by cells that are doubling faster than once per 24 hours,[8] the cyanobacterial rhythms continue in cultures that are growing with doubling times as rapid as one division every 5–6 hours.[9][10][11] Apparently cyanobacteria are able to simultaneously and accurately keep track of two timing processes that express significantly different periods.
Adaptive significance
Do circadian timekeepers enhance the fitness of organisms growing under natural conditions? Despite the expectation that circadian clocks are usually assumed to enhance the fitness of organisms by improving their ability to adapt to daily cycles in environmental factors, there have been few rigorous tests of that proposition in any organism. Cyanobacteria are one of the few organisms in which such a test has been performed. The adaptive fitness test was done by mixing cyanobacterial strains that express different circadian properties (i.e., rhythmicity vs. arhythmicity, different periods, etc.) and growing them in competition under different environmental conditions. The idea was to determine if having an appropriately functional clock system enhances fitness under competitive conditions. The result was that strains with a functioning biological clock out-compete arhythmic strains in environments that have a rhythmic light/dark cycle (e.g., 12 hours of light alternating with 12 hours of darkness), whereas in "constant" environments (e.g., constant illumination) rhythmic and arhythmic strains grow at comparable rates.[12] Among rhythmic strains with different periods, the strains whose endogenous period most closely matches the period of the environmental cycle is able to out-compete strains whose period does not match that of the environment.[13] Therefore, in rhythmic environments, the fitness of cyanobacteria is improved when the clock is operational and when its circadian period is similar to the period of the environmental cycle. These were among the first rigorous demonstrations in any organism of a fitness advantage conferred by a circadian system.
When researchers believed that prokaryotes were too "simple" to have circadian timekeepers, it had seemed reasonable that evolutionary selection for circadian organization would occur only if the generation time of the cells were as long or longer than a day. The data from cyanobacteria, however, suggest that the benefits of having a daily clock can potentially accrue to all organisms, even if they divide more rapidly than once a day.
Global regulation of gene expression and chromosomal topology
In eukaryotes, about 10–20% of the genes are rhythmically expressed (as gauged by rhythms of mRNA abundance). However, in cyanobacteria, a much larger percentage of genes are controlled by the circadian clock. For example, one study has shown that the activity of essentially all promoters is rhythmically regulated.[14] The mechanism by which this global gene regulation is mechanistically linked to the circadian clock is not known, but it may be related to rhythmic changes in the topology of the entire cyanobacterial chromosome.[15][16]
Molecular mechanism of the cyanobacterial clockwork
The S. elongatus luciferase reporter system was used to screen for clock gene mutants, of which many were isolated.[17] These mutants were used to identify a gene cluster composed of three genes, named kaiA, kaiB and kaiC; (Ishiura et al., 1998; "kai" means "rotation" or "cycle number" in Japanese). These genes encode the proteins KaiA, KaiB, and KaiC, which are essential for clock function in S. elongatus and constitute a core circadian oscillator. No significant similarity was found among the kai genes and any other previously reported genes in eukaryotes, but there are potential homologs in the genomic sequences of other bacteria (both eubacteria and archaea).
At first, the cyanobacterial clockwork appeared to be a transcription and translation feedback loop in which clock proteins autoregulate the activity of their own promoters by a process that was similar in concept to the circadian clock loops of eukaryotes.[18]>[19] Subsequently, however, several lines of evidence indicated that transcription and translation was not necessary for circadian rhythms of Kai proteins,[20][21]<re[22] the most spectacular being that the three purified Kai proteins can reconstitute a temperature-compensated circadian oscillation in a test tube.[23] The rhythm that is measurable in vitro is the phosphorylation status of the clock protein KaiC. This is the first (and so far, only) example of the reconstitution of a circadian clock in vitro.
The output of this oscillator to rhythms of gene expression may be mediated by one or both of the following mechanisms: (1) the Biochemical Cascade Model that implicates the globally acting transcription factors, RpaA and B. RpaA seems to be coupled to the central KaiABC oscillator by histidine kinase SasA through a two-component signaling pathway,[24] and/or the (2) Chromosome/Nucleoid Hypothesis, in which the circadian clock orchestrates dramatic circadian changes in DNA topology, which causes a change in the transcription rates.[25][16] The behavior of heterologous promoters from other bacteria when expressed in cyanobacteria support the latter hypothesis.
Visualizing the clockwork's "gears": structural biology of clock proteins
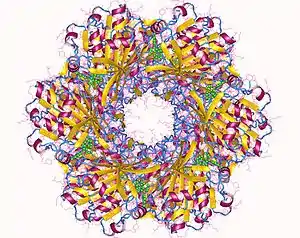
The cyanobacterial circadian system is so far unique in that it is the only circadian system in which the structures of full-length clock proteins have been solved. In fact, the structures of all three of the Kai proteins have been determined. KaiC forms a hexamer that resembles a double doughnut with a central pore that is partially sealed at one end.[26] There are twelve ATP-binding sites in KaiC and the residues that are phosphorylated during the in vitro phosphorylation rhythm have been identified.[27][28] KaiA has two major domains and forms dimers in which the N-terminal domains are "swapped" with the C-terminal domains.[29][30] KaiB has been successfully crystallized from three different species of cyanobacteria and forms dimers or tetramers.[31][32]
The three-dimensional structures have been helpful in elucidating the cyanobacterial clock mechanism by providing concrete models for the ways in which the three Kai proteins interact and influence each other.[26][30][31][33][34][35][36] The structural approaches have also allowed the KaiA/KaiB/KaiC complexes to be visualized as a function of time, which enabled sophisticated mathematical modeling of the in vitro phosphorylation rhythm.[37] Therefore, the cyanobacterial clock components and their interactions can be visualized in four dimensions (three in space, one in time).The temporal formation patterns of the KaiA/KaiB/KaiC complex have been elucidated, along with an interpretation of the core mechanism based on KaiC phosphorylation patterns and the dynamics of the KaiA/KaiB/KaiC complex.[24][38] In addition, single-molecule methods (high-speed atomic force microscopy) have been applied to visualize in real time and quantify the dynamic interactions of KaiA with KaiC on sub-second timescales.[39] These interactions regulate the circadian oscillation by modulating the magnesium binding in KaiC.[40]
An alternative view for the core mechanism of this remarkable clock is based on observations of the ATPase activity of KaiC. KaiC hydrolyses ATP at the remarkably slow rate of only 15 ATP molecules per KaiC monomer per 24 hours. The rate of this ATPase activity is temperature compensated, and the activities of wild-type and period-mutant KaiC proteins are directly proportional to their in vivo circadian frequencies, suggesting that the ATPase activity defines the circadian period. Therefore, some authors have proposed that the KaiC ATPase activity constitutes the most fundamental reaction underlying circadian periodicity in cyanobacteria.[41]
Circadian Advantage
In the context of bacterial circadian rhythms, specifically in cyanobacteria, circadian advantage refers to the improved competitive advantage of strains of cyanobacteria that "resonate" with the environmental circadian rhythm.[13] For example, consider a strain with a free-running period (FRP) of 24 hours that is co-cultured with a strain that has a free-running period (FRP) of 30 hours in a light-dark cycle of 12 hours light and 12 hours dark (LD 12:12). The strain that has a 24-hour FRP will out-compete the 30-hour strain over time under these LD 12:12 conditions. On the other hand, in a light-dark cycle of 15 hours light and 15 hours darkness, the 30-hour strain will out-compete the 24-hour strain.[13] Moreover, rhythmic strains of cyanobacteria will out-compete arhythmic strains in 24-h light/dark cycles, but in continuous light, arhythmic strains are able to co-exist with wild-type cells in mixed cultures.[12]
Other Bacteria
The only prokaryotic group with a well-documented circadian timekeeping mechanism is the cyanobacteria. Recent studies have suggested that there might be 24-hour timekeeping mechanisms among other prokaryotes.[38] The purple non-sulfur bacterium Rhodopseudomonas palustris is one such example, as it harbors homologs of KaiB and KaiC and exhibits adaptive KaiC-dependent growth enhancement in 24-hour cyclic environments.[42] However, R. palustris was reported to show a poor intrinsic free-running rhythm of nitrogen fixation under constant conditions. The lack of rhythm in R. palustris in constant conditions has implications for the adaptive value of intrinsic timekeeping mechanism.[38] Therefore, the R. palustris system was proposed as a “proto” circadian timekeeper that exhibit some parts of circadian systems (kaiB and kaiC homologs), but not all.[42]
Another very interesting example is the case of the microbiome. It is possible that circadian clocks play a role in the gut microbiota behavior. These microorganisms experience daily changes because their hosts eat on a daily routine (consumption in the day for diurnal animals and in the night for nocturnal hosts). The presence of a daily timekeeper might allow gut bacteria to anticipate resources coming from the host temporally, thereby giving those species of bacteria a competitive advantage over other species in the gut.[38] Therefore, the gut microbiota of rhythmically-feeding host is another likely place to look for bacteria that have evolved daily timekeepers.
See also
References
- Johnson CH, Golden SS, Ishiura M, Kondo T (July 1996). "Circadian clocks in prokaryotes". Molecular Microbiology. 21 (1): 5–11. doi:10.1046/j.1365-2958.1996.00613.x. PMID 8843429.
- Huang TC, Grobbelaar N (March 1995). "The circadian clock in the prokaryote Synechococcus RF-1". Microbiology. 141 (3): 535–540. doi:10.1099/13500872-141-3-535.
- Lin RF, Huang TC (2009). "Circadian rhythm of Cyanothece RF-1 (Synechococcus RF-1). Chapter 3". In Ditty JL, Mackey SR, Johnson CH (eds.). Bacterial Circadian Programs. Springer. pp. 39–61.
- Sweeney BM, Borgese MB (1989). "A circadian rhythm in cell division in a prokaryote, the cyanobacterium Synechococcus WH7803". J. Phycol. 25: 183–186. doi:10.1111/j.0022-3646.1989.00183.x.
- Kondo T, Strayer CA, Kulkarni RD, Taylor W, Ishiura M, Golden SS, Johnson CH (June 1993). "Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 90 (12): 5672–6. doi:10.1073/pnas.90.12.5672. PMC 46783. PMID 8516317.
- Johnson CH, Xu Y (2009). "The Decade of Discovery: How Synechococcus elongatus became a model circadian system 1990–2000. Chapter 4". In Ditty JL, Mackey SR, Johnson CH (eds.). Bacterial Circadian Programs. Springer. pp. 63–86.
- Mihalcescu I, Hsing W, Leibler S (July 2004). "Resilient circadian oscillator revealed in individual cyanobacteria". Nature. 430 (6995): 81–5. doi:10.1038/nature02533. PMID 15229601.
- Pittendrigh CS (1993). "Temporal organization: reflections of a Darwinian clock-watcher". Annual Review of Physiology. 55: 16–54. doi:10.1146/annurev.ph.55.030193.000313. PMID 8466172.
- Mori T, Binder B, Johnson CH (September 1996). "Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours". Proceedings of the National Academy of Sciences of the United States of America. 93 (19): 10183–8. doi:10.1073/pnas.93.19.10183. PMC 38358. PMID 8816773.
- Kondo T, Mori T, Lebedeva NV, Aoki S, Ishiura M, Golden SS (January 1997). "Circadian rhythms in rapidly dividing cyanobacteria". Science. 275 (5297): 224–7. doi:10.1126/science.275.5297.224. PMID 8985018.
- Mori T, Johnson CH (April 2001). "Independence of circadian timing from cell division in cyanobacteria". Journal of Bacteriology. 183 (8): 2439–44. doi:10.1128/JB.183.8.2439-2444.2001. PMC 95159. PMID 11274102.
- Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH (August 2004). "The adaptive value of circadian clocks: an experimental assessment in cyanobacteria". Current Biology. 14 (16): 1481–6. doi:10.1016/j.cub.2004.08.023. PMID 15324665.
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH (July 1998). "Resonating circadian clocks enhance fitness in cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 95 (15): 8660–4. doi:10.1073/pnas.95.15.8660. PMC 21132. PMID 9671734.
- Liu Y, Tsinoremas NF, Johnson CH, Lebedeva NV, Golden SS, Ishiura M, Kondo T (June 1995). "Circadian orchestration of gene expression in cyanobacteria". Genes & Development. 9 (12): 1469–78. doi:10.1101/gad.9.12.1469. PMID 7601351.
- Smith RM, Williams SB (May 2006). "Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus". Proceedings of the National Academy of Sciences of the United States of America. 103 (22): 8564–9. doi:10.1073/pnas.0508696103. PMC 1482530. PMID 16707582.
- Woelfle MA, Xu Y, Qin X, Johnson CH (November 2007). "Circadian rhythms of superhelical status of DNA in cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 104 (47): 18819–24. doi:10.1073/pnas.0706069104. PMC 2141860. PMID 18000054.
- Kondo T, Tsinoremas NF, Golden SS, Johnson CH, Kutsuna S, Ishiura M (November 1994). "Circadian clock mutants of cyanobacteria". Science. 266 (5188): 1233–6. doi:10.1126/science.7973706. PMID 7973706.
- Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson CR, Tanabe A, Golden SS, Johnson CH, Kondo T (September 1998). "Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria". Science. 281 (5382): 1519–23. doi:10.1126/science.281.5382.1519. PMID 9727980.
- Dunlap JC, Loros JJ, DeCoursey PJ, eds. (2004). Chronobiology: Biological Timekeeping. Sunderland, MA.: Sinauer.
- Xu Y, Mori T, Johnson CH (May 2003). "Cyanobacterial circadian clockwork: roles of KaiA, KaiB and the kaiBC promoter in regulating KaiC". The EMBO Journal. 22 (9): 2117–26. doi:10.1093/emboj/cdg168. PMC 156062. PMID 12727878.
- Nakahira Y, Katayama M, Miyashita H, Kutsuna S, Iwasaki H, Oyama T, Kondo T (January 2004). "Global gene repression by KaiC as a master process of prokaryotic circadian system". Proceedings of the National Academy of Sciences of the United States of America. 101 (3): 881–5. doi:10.1073/pnas.0307411100. PMC 321775. PMID 14709675.
- Tomita J, Nakajima M, Kondo T, Iwasaki H (January 2005). "No transcription-translation feedback in circadian rhythm of KaiC phosphorylation". Science. 307 (5707): 251–4. doi:10.1126/science.1102540. PMID 15550625.
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T (April 2005). "Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro". Science. 308 (5720): 414–5. doi:10.1126/science.1108451. PMID 15831759.
- Swan JA, Golden SS, LiWang A, Partch CL (April 2018). "Structure, function, and mechanism of the core circadian clock in cyanobacteria". The Journal of Biological Chemistry. 293 (14): 5026–5034. doi:10.1074/jbc.TM117.001433. PMC 5892564. PMID 29440392.
- Vijayan V, Zuzow R, O'Shea EK (December 2009). "Oscillations in supercoiling drive circadian gene expression in cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 106 (52): 22564–8. doi:10.1073/pnas.0912673106. PMC 2799730. PMID 20018699.
- Pattanayek R, Wang J, Mori T, Xu Y, Johnson CH, Egli M (August 2004). "Visualizing a circadian clock protein: crystal structure of KaiC and functional insights". Molecular Cell. 15 (3): 375–88. doi:10.1016/j.molcel.2004.07.013. PMID 15304218.
- Xu Y, Mori T, Pattanayek R, Pattanayek S, Egli M, Johnson CH (September 2004). "Identification of key phosphorylation sites in the circadian clock protein KaiC by crystallographic and mutagenetic analyses". Proceedings of the National Academy of Sciences of the United States of America. 101 (38): 13933–8. doi:10.1073/pnas.0404768101. PMC 518856. PMID 15347809.
- Nishiwaki T, Satomi Y, Nakajima M, Lee C, Kiyohara R, Kageyama H, Kitayama Y, Temamoto M, Yamaguchi A, Hijikata A, Go M, Iwasaki H, Takao T, Kondo T (September 2004). "Role of KaiC phosphorylation in the circadian clock system of Synechococcus elongatus PCC 7942". Proceedings of the National Academy of Sciences of the United States of America. 101 (38): 13927–32. doi:10.1073/pnas.0403906101. PMC 518855. PMID 15347812.
- Williams SB, Vakonakis I, Golden SS, LiWang AC (November 2002). "Structure and function from the circadian clock protein KaiA of Synechococcus elongatus: a potential clock input mechanism". Proceedings of the National Academy of Sciences of the United States of America. 99 (24): 15357–62. doi:10.1073/pnas.232517099. PMC 137721. PMID 12438647.
- Ye S, Vakonakis I, Ioerger TR, LiWang AC, Sacchettini JC (May 2004). "Crystal structure of circadian clock protein KaiA from Synechococcus elongatus". The Journal of Biological Chemistry. 279 (19): 20511–8. doi:10.1074/jbc.M400077200. PMID 15007067.
- Garces RG, Wu N, Gillon W, Pai EF (April 2004). "Anabaena circadian clock proteins KaiA and KaiB reveal a potential common binding site to their partner KaiC". The EMBO Journal. 23 (8): 1688–98. doi:10.1038/sj.emboj.7600190. PMC 394244. PMID 15071498.
- Hitomi K, Oyama T, Han S, Arvai AS, Getzoff ED (May 2005). "Tetrameric architecture of the circadian clock protein KaiB. A novel interface for intermolecular interactions and its impact on the circadian rhythm". The Journal of Biological Chemistry. 280 (19): 19127–35. doi:10.1074/jbc.M411284200. PMID 15716274.
- Vakonakis I, LiWang AC (July 2004). "Structure of the C-terminal domain of the clock protein KaiA in complex with a KaiC-derived peptide: implications for KaiC regulation". Proceedings of the National Academy of Sciences of the United States of America. 101 (30): 10925–30. doi:10.1073/pnas.0403037101. PMC 503721. PMID 15256595.
- Pattanayek R, Williams DR, Pattanayek S, Xu Y, Mori T, Johnson CH, Stewart PL, Egli M (May 2006). "Analysis of KaiA-KaiC protein interactions in the cyano-bacterial circadian clock using hybrid structural methods". The EMBO Journal. 25 (9): 2017–28. doi:10.1038/sj.emboj.7601086. PMC 1456936. PMID 16628225.
- Kim YI, Dong G, Carruthers CW, Golden SS, LiWang A (September 2008). "The day/night switch in KaiC, a central oscillator component of the circadian clock of cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 105 (35): 12825–30. doi:10.1073/pnas.0800526105. PMC 2529086. PMID 18728181.
- Pattanayek R, Williams DR, Pattanayek S, Mori T, Johnson CH, Stewart PL, Egli M (June 2008). "Structural model of the circadian clock KaiB-KaiC complex and mechanism for modulation of KaiC phosphorylation". The EMBO Journal. 27 (12): 1767–78. doi:10.1038/emboj.2008.104. PMC 2435126. PMID 18497745.
- Mori T, Williams DR, Byrne MO, Qin X, Egli M, Mchaourab HS, Stewart PL, Johnson CH (April 2007). "Elucidating the ticking of an in vitro circadian clockwork". PLOS Biology. 5 (4): e93. doi:10.1371/journal.pbio.0050093. PMC 1831719. PMID 17388688.
- Johnson CH, Zhao C, Xu Y, Mori T (April 2017). "Timing the day: what makes bacterial clocks tick?". Nature Reviews. Microbiology. 15 (4): 232–242. doi:10.1038/nrmicro.2016.196. PMC 5696799. PMID 28216658.
- Mori T, Sugiyama S, Byrne M, Johnson CH, Uchihashi T, Ando T (August 2018). "Revealing circadian mechanisms of integration and resilience by visualizing clock proteins working in real time". Nature Communications. 9 (1): 3245. doi:10.1038/s41467-018-05438-4. PMC 6092398. PMID 30108211.
- Jeong, Young M.; Dias, Cristiano; Diekman, Casey; Brochon, Helene; Kim, Pyonghwa; Kaur, Manpreet; Kim, Yong-Sung; Jang, Hye-In; Kim, Yong-Ick (August 2019). "Magnesium Regulates the Circadian Oscillator in Cyanobacteria". Journal of Biological Rhythms. 34 (4): 380–390. doi:10.1177/0748730419851655. ISSN 0748-7304. PMID 31216910.
- Terauchi K, Kitayama Y, Nishiwaki T, Miwa K, Murayama Y, Oyama T, Kondo T (October 2007). "ATPase activity of KaiC determines the basic timing for circadian clock of cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America. 104 (41): 16377–81. doi:10.1073/pnas.0706292104. PMC 2042214. PMID 17901204.
- Ma P, Mori T, Zhao C, Thiel T, Johnson CH (March 2016). "Evolution of KaiC-Dependent Timekeepers: A Proto-circadian Timing Mechanism Confers Adaptive Fitness in the Purple Bacterium Rhodopseudomonas palustris". PLoS Genetics. 12 (3): e1005922. doi:10.1371/journal.pgen.1005922. PMC 4794148. PMID 26982486.
Further reading
- Ditty JL, Mackey SR, Johnson CH, eds. (2009). Bacterial Circadian Programs. Springer. p. 333.
- Dunlap JC, Loros J, DeCoursey PJ (2004). Chronobiology: Biological Timekeeping. Sunderland: Sinauer.