Bacterial nanowires
Bacterial nanowires (also known as microbial nanowires) are electrically conductive appendages produced by a number of bacteria most notably from (but not exclusive to) the Geobacter and Shewanella genera.[1][2] Conductive nanowires have also been confirmed in the oxygenic cyanobacterium Synechocystis PCC6803 and a thermophilic, methanogenic coculture consisting of Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus.[2]
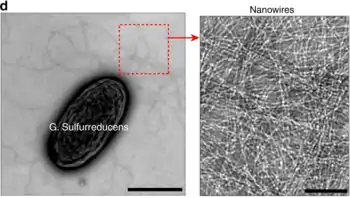
Physiology
Geobacter nanowires were originally thought to be modified pili, which are used to establish connections to terminal electron receptors. Further research has shown that Geobacter nanowires are composed of stacked cytochromes, namely OmcS and OmcZ.[3] These stacked cytochrome nanowires form a seamless array of hemes which stabilize the nanowire via pi-stacking and provide a path for electron transport.[4] Species of the genus Geobacter use nanowires to transfer electrons to extracellular electron acceptors (such as Fe(III) oxides).[5] This function was discovered through the examination of mutants, whose nanowires could attach to the iron, but would not reduce it.[5]
However, Shewanella nanowires are not pili, but extensions of the outer membrane that contain the decaheme outer membrane cytochromes MtrC and OmcA.[6] The reported presence of outer membrane cytochromes, and lack of conductivity in nanowires from the MtrC and OmcA-deficient mutant[7] directly support the proposed multistep hopping mechanism for electron transport through Shewanella nanowires.[8][9][10]
Additionally, nanowires can facilitate long-range electron transfer across thick biofilm layers.[11] By connecting to other cells above them, nanowires allow bacteria located in anoxic conditions to still use oxygen as their terminal electron acceptor. For example, organisms in the genus Shewanella have been observed to form electrically conductive nanowires in response to electron-acceptor limitation.[2]
Implications and potential applications
Biologically it is unclear what is implied by the existence of bacterial nanowires. Nanowires may function as conduits for electron transport between different members of a microbial community.[12]
Bioenergy applications in microbial fuel cells
In microbial fuel cells (MFCs), bacterial nanowires generate electricity via extracellular electron transport to the MFC's anode.[13] Nanowire networks have been shown to enhance the electricity output of MFCs with efficient and long-range conductivity. In particular, pili of Geobacter sulfurreducens possess metallic-like conductivity, producing electricity at levels comparable to those of synthetic metallic nanostructures.[14] When bacterial strains are genetically manipulated to boost nanowire formation, higher electricity yields are generally observed.[15] Coating the nanowires with metal oxides also further promotes electrical conductivity.[16] Additionally, these nanowires can transport electrons up to centimetre-scale distances.[15] Long-range electron transfer via pili networks allows viable cells that are not in direct contact with an anode to contribute to electron flow.[17] Thus, increased current production in MFCs is observed in thicker biofilms.
The currency produced by bacterial nanowires very low. Current density of around 17 microamperes per square centimetre, voltage of around 0.5 volts across a 7-micrometre-thick film.[18]
Other significant applications
Bacterial nanowires have been shown to have significant potential applications in the fields of bioenergy and bioremediation.[19] Electron transfer between the pili of Geobacter, a dissimilatory metal-reducing bacterium, generates conductivity that drives the conversion of organic compounds to electricity in microbial fuel cells.[20] Biofilms produced by Geobacter colonies contribute greatly to the overall production of bioenergy. They allow the transfer of electrons via conductive pili over a greater distance from the anode.[19] In fact, Bioenergy output can be further enhanced by inducing the expression of additional nanowire genes. Geobacter strains with heightened expression of conductive pili have been shown to produce more conductive biofilms, thus increasing overall electricity output.[20]
Microbial nanowires of Shewanella and Geobacter have also been shown to aid in bioremediation of uranium contaminated groundwater.[21] To demonstrate this, scientists compared and observed the concentration of uranium removed by piliated and nonpiliated strains of Geobacter. Through a series of controlled experiments, they were able to deduce that nanowire present strains were more effective at the mineralization of uranium as compared to nanowire absent mutants.[22]
Further application significance of bacterial nanowires include bioelectronics.[19] With sustainable resources in mind, scientists have proposed the future use of biofilms of Geobacter as a platform for functional under water transistors and supercapacitors, capable of self-renewing energy.[23]
On 20 April 2020 researchers demonstrated a diffusive memristor fabricated from protein nanowires of the bacterium Geobacter sulfurreducens which functions at substantially lower voltages than the ones previously described and may allow the construction of artificial neurons which function at voltages of biological action potentials. The nanowires have a range of advantages over silicon nanowires and the memristors may be used to directly process biosensing signals, for neuromorphic computing and/or direct communication with biological neurons.[24][25][26]
References
- Reguera, G; McCarthy, KD; Mehta, T; Nicoll, JS; Tuominen, MT; Lovley, DR (2005). "Extracellular electron transfer via microbial nanowires". Nature. 435: 1098–101. doi:10.1038/nature03661. PMID 15973408.
- Yuri A. Gorby; Svetlana Yanina; Jeffrey S. McLean; Kevin M. Rosso; Dianne Moyles; Alice Dohnalkova; Terry J. Beveridge; In Seop Chang; Byung Hong Kim; Kyung Shik Kim; David E. Culley; Samantha B. Reed; Margaret F. Romine; Daad A. Saffarini; Eric A. Hill; Liang Shi; Dwayne A. Elias; David W. Kennedy; Grigoriy Pinchuk; Kazuya Watanabe; Shun’ichi Ishii; Bruce Logan; Kenneth H. Nealson & Jim K. Fredrickson (2006). "Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms". Proceedings of the National Academy of Sciences. 103 (30): 11358–11363. doi:10.1073/pnas.0604517103. PMC 1544091. PMID 16849424.
- Sibel; et al. (2020). "Electric field stimulates production of highly conductive microbial OmcZ nanowires". Nature Chemical Biology. doi:10.1038/s41589-020-0623-9. Retrieved 30 September 2020.
- Wang, Fenbin (2019). "Structure of Microbial Nanowires Reveals Stacked Hemes that Transport Electrons over Micrometers". Cell. doi:10.1016/j.cell.2019.03.029. Retrieved 30 September 2020.
- Reguera; et al. (2005). "Extracellular electron transfer via microbial nanowires". Nature. 435 (7045): 1098–1101. doi:10.1038/nature03661. PMID 15973408.
- Pirbadian; et al. (2014). "Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components". Proc Natl Acad Sci USA. 111 (35): 12883–12888. doi:10.1073/pnas.1410551111. PMC 4156777. PMID 25143589.
- El-Naggar; et al. (2010). "Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1". Proc Natl Acad Sci USA. 107 (42): 18127–18131. doi:10.1073/pnas.1004880107. PMC 2964190. PMID 20937892.
- Pirbadian, S.; El-Naggar, M.Y. (2012). "Multistep hopping and extracellular charge transfer in microbial redox chains". Phys Chem Chem Phys. 14 (40): 13802–8. doi:10.1039/C2CP41185G. PMID 22797729.
- Polizzi, NF; et al. (2012). "Physical constraints on charge transport through bacterial nanowires". Faraday Discuss. 155: 43–61. doi:10.1039/C1FD00098E. PMC 3392031.
- Strycharz-Glaven, SM; et al. (2011). "On the electrical conductivity of microbial nanowires and biofilms". Energy Environ Sci. 4 (11): 4366–4379. doi:10.1039/C1EE01753E.
- Reguera; et al. (2006). "Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells". Appl. Environ. Microbiol. 72 (11): 7345–8. doi:10.1128/aem.01444-06. PMC 1636155. PMID 16936064.
- Rabaey, Korneel; Rozendal, René A. (2010). "Microbial electrosynthesis — revisiting the electrical route for microbial production". Nature Reviews Microbiology. 8 (10): 706–716. doi:10.1038/nrmicro2422. ISSN 1740-1526. PMID 20844557.
- Kodesia, A.; Ghosh, M.; Chatterjee, A. (September 5, 2017). "Development of Biofilm Nanowires and Electrode for Efficient Microbial Fuel Cells (MFCs)". Thapar University Digital Repository (TuDR).
- Malvankar, N.S.; Vargas, M.; Nevin, K.P.; Franks, A.E.; Leang, C.; Kim, B.C.; Inoue, K. (August 7, 2011). "Tunable metallic-like conductivity in microbialnanowire networks". Nature Nanotechnology. 6: 573–579. doi:10.1038/NNANO.2011.119.
- Malvankar, N.S.; Lovley, D.R. (May 21, 2012). "Microbial Nanowires: A New Paradigm for Biological Electron Transfer and Bioelectronics". ChemSusChem. 5 (6): 1039–1046. doi:10.1002/cssc.201100733 – via Wiley Online Library.
- Maruthupandy, M.; Anand, M.; Maduraiveeran, G. (June 5, 2017). "Fabrication of CuO nanoparticles coated bacterial nanowire film for a high-performance electrochemical conductivity". J Mater Sci. 52: 10766–10778. doi:10.1007/s10853-017-1248-6.
- Reguera, Gemma (2006). "Biofilm and Nanowire Production Leads to Increased Current in Geobacter sulfurreducens Fuel Cells". Appl. Environ. Microbiol. 72: 7345–7348. doi:10.1128/aem.01444-06. PMC 1636155. PMID 16936064.
- Xiaomeng Liu, Hongyan Gao, Joy E. Ward, Xiaorong Liu, Bing Yin, Tianda Fu, Jianhan Chen, Derek R. Lovley & Jun Yao (2020). "Power generation from ambient humidity using protein nanowires". Nature. 578 (7796): 550–554. doi:10.1038/s41586-020-2010-9. PMID 32066937.CS1 maint: multiple names: authors list (link)
- Sure, Sandeep; Ackland, M. Leigh; Torriero, Angel A. J.; Adholeya, Alok; Kochar, Mandira (2016). "Microbial nanowires: an electrifying tale". Microbiology. 162 (12): 2017–2028. doi:10.1099/mic.0.000382. PMID 27902405.
- Malvankar, Nikhil S; Lovley, Derek R (1 June 2014). "Microbial nanowires for bioenergy applications". Current Opinion in Biotechnology. 27: 88–95. doi:10.1016/j.copbio.2013.12.003. PMID 24863901.
- Jiang, Shenghua; Kim, Min-Gyu; Kim, Soo-Jin; Jung, Hyun Suk; Lee, Su Woong; Noh, Do Young; Sadowsky, Michael J.; Hur, Hor-Gil (5 July 2011). "Bacterial formation of extracellularU(VI) nanowires". Chemical Communications. 47 (28): 8076–8. doi:10.1039/C1CC12554K. PMID 21681306.
- Cologgi, Dena L.; Lampa-Pastirk, Sanela; Speers, Allison M.; Kelly, Shelly D.; Reguera, Gemma (13 September 2011). "Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism". Proceedings of the National Academy of Sciences. 108 (37): 15248–15252. doi:10.1073/pnas.1108616108. PMC 3174638. PMID 21896750.
- Malvankar, Nikhil S.; Lovley, Derek R. (1 June 2012). "Microbial Nanowires: A New Paradigm for Biological Electron Transfer and Bioelectronics". ChemSusChem. 5 (6): 1039–1046. doi:10.1002/cssc.201100733. PMID 22614997.
- "Scientists create tiny devices that work like the human brain". The Independent. 20 April 2020. Retrieved 17 May 2020.
- "Researchers unveil electronics that mimic the human brain in efficient learning". phys.org. Retrieved 17 May 2020.
- Fu, Tianda; Liu, Xiaomeng; Gao, Hongyan; Ward, Joy E.; Liu, Xiaorong; Yin, Bing; Wang, Zhongrui; Zhuo, Ye; Walker, David J. F.; Joshua Yang, J.; Chen, Jianhan; Lovley, Derek R.; Yao, Jun (20 April 2020). "Bioinspired bio-voltage memristors". Nature Communications. 11 (1): 1–10. doi:10.1038/s41467-020-15759-y. ISSN 2041-1723. Retrieved 17 May 2020.