Benzenehexol
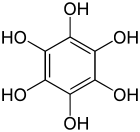
Benzenehexol, also called hexahydroxybenzene, is an organic compound with formula C6H6O6 or C6(OH)6. It is a six-fold phenol of benzene.[2][3] The product is also called hexaphenol,[4] but this name has been used also for other substances.[5]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Benzenehexol[1] | |
| Other names
Benzene-1,2,3,4,5,6-hexol Hexahydroxybenzene 2,3,4,5,6-pentahydroxyphenol 1,2,3,4,5,6-hexahydroxybenzene Hexaphenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.204.877 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6O6 | |
| Molar mass | 174.11 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzenehexol is a crystalline solid soluble in hot water,[4] with a melting point above 310°.[2] It can be prepared from inositol (cyclohexanehexol). Oxidation of benzenehexol yields tetrahydroxy-p-benzoquinone (THBQ), rhodizonic acid, and dodecahydroxycyclohexane.[6] Conversely, benzenehexol can be obtained by reduction of sodium THBQ salt with SnCl2/HCl.[7]
Benzenehexol is a starting material for a class of discotic liquid crystals.[7]
Benzenehexol forms an adduct with 2,2'-bipyridine, with 1:2 molecular ratio.[8]
Benzenehexolate
Like most phenols, benzenehexol can lose the six H+ ions from the hydroxyl groups, yielding the hexaanion C
6O6−
6. The potassium salt of this anion is one of the components of Liebig's so-called "potassium carbonyl", the product of the reaction of carbon monoxide with potassium. The hexaanion is produced by trimerization of the acetylenediolate anion C
2O2−
2 when heating potassium acetylenediolate K
2C
2O
2.[9] The nature of K
6C
6O
6 was clarified by R. Nietzki and T. Benckiser in 1885, who found that its hydrolysis yielded benzenehexol.[10][11][12]
The lithium salt of this anion, Li6C6O6 has been considered for electric battery applications.[13]
Esters
Hexahydroxy benzene forms esters such as the hexaacetate C
6(-O(CO)CH3)6 (melting point 220 °C) and ethers like hexa-tert-butoxybenzene C
6(-OC(CH3)3)6 (melting point 223 °C).[9]
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 693. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- A. J. Fatiadi and W. F. Sager (1973), Hexahydroxybenzene [Benzenehexol] Organic Syntheses, Coll. Vol. 5, p. 595
- Gerd Leston(1996), (Polyhydroxy)benzenes. In Kirk‑Othmer Encyclopedia of Chemical Technology, John Wiley & Sons. doi:10.1002/0471238961.1615122512051920.a01
- J.I.G. Codagan, John Buckingham, Finlay J. MacDonald, P. H. Rhodes (1996), Dictionary of organic compounds. CRC Press. 9000 pages. ISBN 0-412-54090-8, ISBN 978-0-412-54090-5.
- HEXAPHENOL Basic information. Chemical Book. Accessed on 2009-07-05.
- Alexander J. Fatiadi; Horace S. Isbell; William F. Sager (March–April 1963). "Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol)" (PDF). Journal of Research of the National Bureau of Standards Section A. 67A (2): 153–162. doi:10.6028/jres.067A.015. PMC 6640573. PMID 31580622. Archived from the original (PDF) on 2009-03-25. Retrieved 2009-07-05.
- Kumar Sandeep (2006). "Self-organization of disc-like molecules: chemical aspects". Chem. Soc. Rev. 35 (1): 83–109. doi:10.1039/b506619k.
- Cowan John A., Howard Judith A. K., Leech Michael A., Puschmann Horst, Williams Ian D. (2001). "Hexahydroxybenzene—2,2'-bipyridine (1/2)". Acta Crystallographica Section C. 57 (10): 1194–1195. doi:10.1107/S0108270101011350.CS1 maint: multiple names: authors list (link)
- Serratosa Fèlix (1983). "Acetylene Diethers: A Logical Entry to Oxocarbons". Acc. Chem. Res. 16 (5): 170–176. doi:10.1021/ar00089a004.
- R. Nietzki and T. Benckiser (1885), Berichte Chemie, volume 18, page 1834. Cited by Fatiadi and Sanger.
- Ludwig Mond (1892), On metallic carbonyls. Proceedings of the Royal Institution, volume 13, pages 668-680. Reprinted in The Development of Chemistry, 1789-1914: Selected essays edited by D. Knight (1998). ISBN 0-415-17912-2 Online version at books.google.com, accessed on 2010-01-15.
- Büchner Werner, Weiss E (1964). "Zur Kenntnis der sogenannten "Alkalicarbonyle" IV[1] Über die Reaktion von geschmolzenem Kalium mit Kohlenmonoxid". Helvetica Chimica Acta. 47 (6): 1415–1423. doi:10.1002/hlca.19640470604.
- Chen Haiyan, Armand Michel, Courty Matthieu, Jiang Meng, Grey Clare P., Dolhem Franck, Tarascon Jean-Marie, Poizot Philippe (2009). "Lithium Salt of Tetrahydroxybenzoquinone: Toward the Development of a Sustainable Li-Ion Battery". J. Am. Chem. Soc. 131 (25): 8984–8988. doi:10.1021/ja9024897. PMID 19476355.CS1 maint: multiple names: authors list (link)