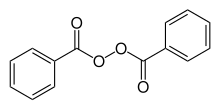
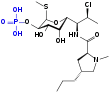Benzoyl peroxide/clindamycin
Benzoyl peroxide/clindamycin is a topical gel used for the treatment of acne. It is a combination of clindamycin, an antibiotic, and benzoyl peroxide, an antiseptic. All formulations are prescription drugs.
| |||
| Combination of | |||
|---|---|---|---|
| Benzoyl peroxide | Antiseptic | ||
| Clindamycin phosphate | Antibiotic | ||
| Clinical data | |||
| Trade names | Duac, BenzaClin, Onexton, Acanya, others | ||
| AHFS/Drugs.com | benzaclin | ||
| MedlinePlus | a603021 | ||
| License data |
| ||
| Routes of administration | Topical (gel) | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Identifiers | |||
| PubChem SID | |||
| KEGG | |||
| | |||
Common side effects include peeling, itching, and dryness of the skin where the gel was applied.
Medical uses
The gel is used on the skin to treat light to medium acne vulgaris in people 12 years and older.[1][2] BenzaClin is to be applied twice daily,[3] and Duac and Onexton are once-a-day medications. Continued use beyond 12-weeks has not been studied.[2]
Efficacy
There is an average 52% decrease in inflammatory acne lesions by week 12.[4]
The combination less is effective than benzoyl peroxide/salicylic acid after short-term treatment of two to four weeks, but the two treatments showed similar effectiveness after ten to twelve weeks.[5]
Pregnancy and lactation
Currently, studies on whether or not the use of benzoyl peroxide/clindamycin gels is teratogenic or has adverse effects on nursing infants has not been performed. While oral clindamycin passes into breast milk, no such data are available for clindamyin in gel form. Limited data regarding topical benzoyl peroxide and clindamycin have shown no safety problems.[1][3]
Contraindications
The gel is not recommended for those who are allergic to clindamycin, benzoyl peroxide, any components of the formulation, or lincomycin.[1][2] Individuals previously diagnosed with regional enteritis, ulcerative colitis, or antibiotic-associated colitis are also recommended not to use it.[6]
Side effects
Common side effects are peeling, itching, redness, dryness, burning, and dermatitis. Benzoyl peroxide bleaches hair, clothes, towels, bedclothing, and the like. Prolonged exposure to natural or artificial sun light (UV rays) is not recommended because the gel may cause photosensitivity.[1][2] Irritation due to benzoyl peroxide can be reduced by avoiding harsh facial cleansers and wearing sunscreen prior to sun exposure.[7]
Clinical studies have shown systemic absorption of clindamycin through topical application, in some cases leading to diarrhea, bloody diarrhea, and colitis. Reports of anaphylaxis were also seen. However, the sources of these reports were personal accounts without controls and of an unknown population, thus it is difficult to attribute their cause to the benzoyl peroxide/clindamycin gel.[2]
Interactions
No formal interaction studies have been done. Combination with topical products containing alcohol or astringents, as well as skin peelings, may increase the irritant effect of benzoyl peroxide/clindamycin. Topical erythromycin may antagonise the effect of clindamycin, although this has only be demonstrated in in vitro studies. Topical tretinoin and other retinoids may be inactivated by benzoyl peroxide or increase its irritant effect.[1][2]
Pharmacology
Mechanism of action
Clindamycin phosphate is a water-soluble ester of the semi-synthetic antibiotic clindamycin, which is synthesized from lincomycin. Like the macrolide antibiotics, it acts as a bacteriostatic agent by interfering with the 50S subunit of the ribosome of Cutibacterium acnes, inhibiting bacterial protein synthesis and preventing bacteria from replicating.[1][8] C. acnes plays a role in the development of acne.[9]
Benzoyl peroxide also kills C. acnes, but by releasing free radical oxygen species, thus oxidizing bacterial proteins. Also, it dries out the area by reducing sebum production, prevents clogged pores, and is a keratolytic agent.[1] Since benzoyl peroxide is an oxidizer, not an antibiotic, it is not subject to C. acnes resistance unlike clindamycin.[5][10]
Both ingredients have been shown to reduce the number of acne lesions with statistical significance.[5]
Pharmacokinetics
Clindamycin phosphate is an inactive prodrug. It is quickly activated to clindamycin by hydrolysis. After four weeks of application during a study, 0.043% of the used clindamycin dose were found in the blood. Benzoyl peroxide is only absorbed through the skin after reduction to benzoic acid, which is subsequently metabolized to hippuric acid and eliminated via the kidneys.[1]
Society and culture
Brand names
There are several branded drugs approved by the FDA. The first is Acanya, a 1.2% clindamycin phosphate and 2.5% benzoyl peroxide; though because Valeant acquired the rights to Acanya when it bought out Dow Pharmaceutical Science in 2009, it should not be considered a competitor.[11] The other drug, Duac, a 1.2% clindamycin phosphate ad 5% benzoyl peroxide, was approved by the FDA on 26 August 2002.[12] BenzaClin, another gel, has the same concentrations as Duac.[3]
Patent
Dow Pharmaceuticals filed the patent for Onexton, and the United States Patent and Trademark Office issued the patent on 16 October 2012. On 24 November 2014, the US Food and Drug Administration (FDA) approved the new drug application (NDA) No. 050819 for Onexton, with Dow Pharmaceutical as the holder.[13] The patent is set to expire on 5 August 2029.[12]
Lawsuits
On 12 January 2016,[14] Dow Pharmaceutical Sciences and Valeant Pharmaceuticals North America LLC filed a lawsuit against Taro USA and Taro Industries, an Israel-based corporation. The lawsuit was filed for infringement upon their Onexton patent, by Taro attempting to submit an abbreviated new drug application (ANDA) to the FDA for its generic 3.75% benzoyl peroxide and 1.2% clindamycin phosphate topical gel. The court concluded that Taro was guilty of attempting to submit an ANDA patent request before the Onexton patent expired.[13]
References
- Haberfeld H, ed. (2016). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Indoxyl 10 mg/g + 50 mg/g Gel.
- "Onexton (Valeant Pharmaceuticals North America LLC): FDA Package Insert". MedLibrary.org. Retrieved 2016-11-19.
- FDA Professional Drug Information on BenzaClin. Retrieved 2017-01-25.
- "Onexton gel for acne". The Medical Letter on Drugs and Therapeutics. 57 (1476): 124–5. August 2015. PMID 26305525.
- Seidler EM, Kimball AB (July 2010). "Meta-analysis comparing efficacy of benzoyl peroxide, clindamycin, benzoyl peroxide with salicylic acid, and combination benzoyl peroxide/clindamycin in acne". Journal of the American Academy of Dermatology. 63 (1): 52–62. doi:10.1016/j.jaad.2009.07.052. PMID 20488582.
- "Onexton (Cindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/3.75) Drug Information: Overdosage and Contraindications - Prescribing Information at RxList". RxList. Retrieved 2016-11-19.
- Koda-Kimble MA, Alldredge BK, eds. (2013). Applied Therapeutics: The Clinical Use of Drugs (10th ed.). Baltimore: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 949. ISBN 978-1609137137.
- "Lincosamides, Oxazolidinones, and Streptogramins". Merck Manual of Diagnosis and Therapy. Merck & Co. November 2005. Retrieved 2007-12-01.
- Kim J (2005). "Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses". Dermatology. 211 (3): 193–8. doi:10.1159/000087011. PMID 16205063. S2CID 21459334.
- Simonart T (December 2012). "Newer approaches to the treatment of acne vulgaris". American Journal of Clinical Dermatology. 13 (6): 357–64. doi:10.2165/11632500-000000000-00000. PMID 22920095.
- "Key Facts and History". www.valeant.com. Retrieved 2016-11-19.
- Approved Drug Products With Therapeutic Equivalence Evaluations (36th ed.). U.S. Food and Drug Administration. 19 November 2016.
- Dow Pharmaceutical Sciences, Inc. and Valeant Pharmaceuticals North America LLC v. Taro Pharmaceuticals U.S.A., INC. and Taro Pharmaceutical Industries LTD. United States District Court District of New Jersey. 01 Dec. 2016. Print.
- Chuderewicz MA. "Case 2:16-cv-00217-SRC-CLW".