Beta-lactamase inhibitor protein
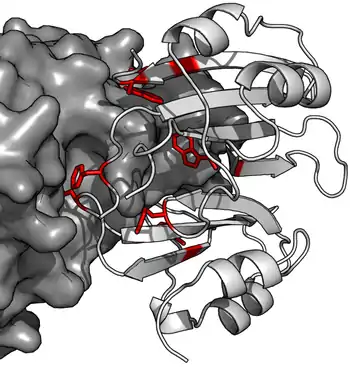
Beta-Lactamase Inhibitor Proteins (BLIPs) are a family of proteins produced by bacterial species including Streptomyces. BLIP acts as a potent inhibitor of beta-lactamases such as TEM-1, which is the most widespread resistance enzyme to penicillin antibiotics. BLIP binds competitively the surface of TEM-1 and inserting residues into the active site to make direct contacts with catalytic residues. BLIP is able to inhibit a variety of class A beta-lactamases, possibly through flexibility of its two domains. The two tandemly repeated domains of BLIP have an α2-β4 structure, the β-hairpin loop from domain 1 inserting into the active site of beta-lactamase.[1] BLIP shows no sequence similarity with BLIP-II, even though both bind to and inhibit TEM-1.[2]
| Beta-Lactamase Inhibitor Protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | BLIP | ||||||||
| Pfam | PF07467 | ||||||||
| Pfam clan | CL0320 | ||||||||
| InterPro | IPR009099 | ||||||||
| SCOP2 | 1s0w / SCOPe / SUPFAM | ||||||||
| |||||||||
References
- Strynadka NC, Jensen SE, Alzari PM, James MN (March 1996). "A potent new mode of beta-lactamase inhibition revealed by the 1.7 A X-ray crystallographic structure of the TEM-1-BLIP complex". Nat. Struct. Biol. 3 (3): 290–7. doi:10.1038/nsb0396-290. PMID 8605632. S2CID 22870717.
- Lim D, Park HU, De Castro L, Kang SG, Lee HS, Jensen S, Lee KJ, Strynadka NC (October 2001). "Crystal structure and kinetic analysis of beta-lactamase inhibitor protein-II in complex with TEM-1 beta-lactamase". Nat. Struct. Biol. 8 (10): 848–52. doi:10.1038/nsb1001-848. PMID 11573088. S2CID 29753789.