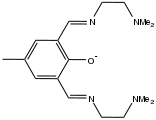
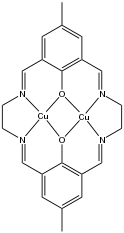
Binucleating ligand
In coordination chemistry, a binucleating ligand binds two metals. Much attention has been directed toward such ligands that hold metals side-by-side, such that the pair of metals can bind substrates cooperatively.[1]
A variety of metalloenzymes feature bimetallic active sites. Examples include superoxide dismutase, urease, nickel-iron hydrogenase. Many Non-heme iron proteins have diiron active sites, e.g. ribonucleotide reductase and hemerythrin.
Examples
Usually binucleating ligands feature bridging ligands, such as phenoxide, pyrazolate, or pyrazine, as well as other donor groups that bind to only one of the two metal ions. Some ligands binucleating ligands are symmetrical, which facilitates the formation of homobimetallic complexes. Other binucleating ligands, where the binding compartments are dissimilar, facilitate the formation of heterobimetallic complexes.
References
- Gavrilova, A. L.; Bosnich, B., "Principles of Mononucleating and Binucleating Ligand Design", Chem. Rev. 2004, volume 104, 349-383. doi:10.1021/cr020604g
- Blake, Antony B.; Fraser, Louis R. (1974). "Crystal Structure and Magnetic Properties of a Binuclear Triketonate Complex of Five-Co-ordinate Copper(II): Bis[heptanetrionato(2–)]bis-pyridinedicopper(II)". J. Chem. Soc., Dalton Trans. (23): 2554–2558. doi:10.1039/dt9740002554.
- Adams, Harry; Clunas, Scott; Fenton, David E. (2004). "A Dinuclear Nickel(II) Complex of the Ligand 2,6-bis[2-(dimethylamino)ethyliminomethyl]-4-methylphenol". Acta Crystallographica Section e Structure Reports Online. 60 (3): m338–m339. doi:10.1107/S1600536804004118.
- Bodman, Samantha E.; Fitchett, Christopher M. (2014). "Conformational Control of the Self-Assembly of Triple Helicates and [2 × 2]-Grids from Zinc(II) and 3,6-Di(2-pyridyl)pyridazine Based Ligands". Dalton Transactions. 43 (33): 12606–13. doi:10.1039/C4DT01416B. PMID 25005269.