Biopterin
Biopterins are pterin derivatives which function as endogenous enzyme cofactors in many species of animals and in some bacteria and fungi. Biopterins act as cofactors for aromatic amino acid hydroxylases (AAAH), which are involved in the synthesis of a number of neurotransmitters including dopamine, norepinephrine, epinepherine, and serotonin, along with several trace amines. Nitric oxide synthesis also uses biopterin derivatives as cofactors. In humans, tetrahydrobiopterin is the endogenous cofactor for AAAH enzymes.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
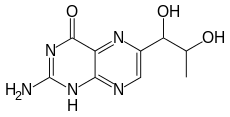
2-Amino-6-(1,2-dihydroxypropyl)-1H-pteridin-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.040.719 |
| KEGG | |
| MeSH | Biopterin |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H11N5O3 | |
| Molar mass | 237.216 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Compounds
Biopterin compounds found within the body include BH4, the free radical[1] BH3, and BH2 (also a free radical).
Synthesis
Biopterin synthesis occurs through two principal pathways; the de novo pathway involves three enzymatic steps and proceeds from GTP, while the salvage pathway converts sepiapterin to biopterin.[2] BH4 is the principal active cofactor, and a recycling pathway converts BH2 to BH4.
Biopterin disorders
A number of disorders of biopterin regulation exist.
Single-gene defects affecting the gene GCH1 block the first step in biopterin synthesis, and lead to dopamine-responsive dystonia, also known as Segawa's syndrome. This is due to the role of BH4 in synthesising neurotransmitters, including Dopamine, and is treated with supplementation with levodopa, which does not require BH4 for conversion to dopamine. GCH1 defects are autosomal dominant, meaning that only one defective gene copy is required for the condition to occur. Mouse gene knockout models that block biopterin synthesis completely die shortly after birth due to their inability to produce catecholamines and neurotransmitters.[3]
Biopterin synthesis disorders are also a cause of hyperphenylalaninemia; phenylalanine metabolism requires BH4 as a cofactor.[4]
References
- Kuzkaya, N.; Weissmann, N.; Harrison, D. G.; Dikalov, S. (2003). "Interactions of Peroxynitrite, Tetrahydrobiopterin, Ascorbic Acid, and Thiols: Implications For Uncoupling Endothelial Nitric-Oxide Synthase". Journal of Biological Chemistry. 278 (25): 22546–54. doi:10.1074/jbc.M302227200. PMID 12692136.
- Nichol, C. A.; Lee, C. L.; Edelstein, M. P.; Chao, J. Y.; Duch, D. S. (1983). "Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo". Proceedings of the National Academy of Sciences of the United States of America. 80 (6): 1546–50. doi:10.1073/pnas.80.6.1546. PMC 393638. PMID 6572916.
- Elzaouk, L; Leimbacher, W; Turri, M; Ledermann, B; Burki, K; Blau, N; Thony, B (2003). "Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in Pts-/- mice rescued by feeding neurotransmitter precursors and H4-biopterin". Journal of Biological Chemistry. 278 (30): 28303–11. doi:10.1074/jbc.M303986200. PMID 12734191.
- "Archived copy". Archived from the original on 2007-06-29. Retrieved 2007-06-07.CS1 maint: archived copy as title (link)
External links
WiseGeek. (2012). What is biopterin?. Retrieved from http://www.wisegeek.com/what-is-biopterin.htm