Callose
Callose is a plant polysaccharide. Its production is due to the glucan synthase-like gene (GLS) in various places within a plant. It is produced to act as a temporary cell wall in response to stimuli such as stress or damage.[1] Callose is composed of glucose residues linked together through β-1,3-linkages, and is termed a β-glucan. It is thought to be manufactured at the cell wall by callose synthases and is degraded by β-1,3-glucanases. Callose is very important for the permeability of plasmodesmata (Pd) in plants; the plant’s permeability is regulated by plasmodesmata callose (PDC). PDC is made by callose synthases and broken down by β-1,3-glucanases (BGs). The amount of callose that is built up at the plasmodesmatal neck, which is brought about by the interference of callose synthases (CalSs) and β-1,3-glucanases, determines the conductivity of the plasmodesmata.[2]
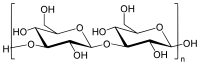 | |
| Names | |
|---|---|
| Other names
(1→3)-β-D-Glucan | |
| Identifiers | |
| ChEBI | |
| Properties | |
| (C6H10O5)n | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Formation and function
Callose is laid down at plasmodesmata, at the cell plate during cytokinesis, and during pollen development. Endothecium contains a substance callose, which makes it thicker. Callose is produced in response to wounding, infection by pathogens,[3] aluminium, and abscisic acid. When there is wounding in the plant tissue, it is fixed by the deposition of callose at the plasmodesmata and cell wall; this process happens within minutes after damage. Even though callose is not a constitutional component of the plant’s cell wall, it is related to the plant’s defense mechanism.[4] Deposits often appear on the sieve plates at the end of the growing season.[5] Callose also forms immediately around the developing meiocytes and tetrads of sexually reproducing angiosperms but is not found in related apomictic taxa.[6] Callose deposition at the cell wall has been suggested as an early marker for direct somatic embryogenesis from cortical and epidermal cells of Cichorium hybrids.[7] Temporary callose walls are also thought to be a barrier between a cell and its environment, while the cell is undergoing a genetic programming that allows it to differentiate.[8] This is because, callose walls can be found around nucellar embryos during Nucellar embryony.[9]
See also
References
- Hine R, Martin E, eds. (2016). "Callose". A Dictionary of Biology. Oxford University Press.
- De Storme N, Geelen D (2014). "Callose homeostasis at plasmodesmata: molecular regulators and developmental relevance". Frontiers in Plant Science. 5: 138. doi:10.3389/fpls.2014.00138. PMC 4001042. PMID 24795733.
- Nowicki M, Lichocka M, Nowakowska M, Kłosińska U, Kozik EU (January 2012). "A Simple Dual Stain for Detailed Investigations of Plant-Fungal Pathogen Interactions". Vegetable Crops Research Bulletin. 77 (1): 61–74. doi:10.2478/v10032-012-0016-z.
- Chen XY, Kim JY (June 2009). "Callose synthesis in higher plants". Plant Signaling & Behavior. 4 (6): 489–92. doi:10.4161/psb.4.6.8359. PMC 2688293. PMID 19816126.
- Hemsley AR, Bell PR (2000). Green plants : their origin and diversity (2nd ed.). Cambridge: Cambridge University Press. ISBN 978-0-521-64109-8.
- Carman JG, Crane CF, Riera-Lizarazu O (1991). "Comparative Histology of Cell Walls during Meiotic and Apomeiotic Megasporogenesis in Two Hexaploid Australasian Elymus Species". Crop Science. 31 (6): 1527. doi:10.2135/cropsci1991.0011183X003100060029x.
- Dubois T, Guedira M, Dubois J, Vasseur J (May 1990). "Direct Somatic Embryogenesis in Roots of Cichorium: Is Callose an Early Marker?". Annals of Botany. 65 (5): 539–545. doi:10.1093/oxfordjournals.aob.a087967.
- Tucker MR, Paech NA, Willemse MT, Koltunow AM (2001). "Dynamics of callose deposition and ß-1, 3-glucanase expression during reproductive events in sexual and apomictic Hieracium". Planta. 212 (4): 487–498. doi:10.1007/s004250000445. PMID 11525505. S2CID 12073031.
- Gupta P, Shivanna KR, Mohan Ram HY (1996). "Apomixis and polyembryony in the guggul plant, Commiphora wightii". Ann Bot. 78: 67–72. doi:10.1006/anbo.1996.0097.