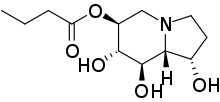
Celgosivir
Celgosivir, in development by Migenix for the treatment of hepatitis C virus (HCV) infection, is an oral prodrug of the natural product castanospermine that inhibits alpha-glucosidase I, an enzyme that plays a critical role in viral maturation by initiating the processing of the N-linked oligosaccharides of viral envelope glycoproteins. Celgosivir is well absorbed in vitro and in vivo, and is rapidly converted to castanospermine. Celgosivir has a novel mechanism of action (preventing the glycosylation of viral proteins by the host), and demonstrates broad antiviral activity in vitro.[1]
 | |
| Clinical data | |
|---|---|
| Other names | 6-O-Butanoylcastanospermine; MDL-28574; MX-3253 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H21NO5 |
| Molar mass | 259.302 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clinical trials
Celgosivir is not efficient as a monotherapy for the treatment of HCV, but has demonstrated a synergistic effect in combination with pegylated interferon alfa-2b plus ribavirin, both in vitro and in phase II clinical trials that last up to 1 year in patients with chronic HCV infection. Celgosivir may prove to be a valuable component for combination therapy and may help to prevent the apparition of drug resistance. Long-term toxicity studies are necessary to confirm the safety of celgosivir in humans.[1]
Although generally safe and well tolerated, celgosivir does not seem to reduce viral load or fever burden in patients with dengue fever.[2]
References
- Durantel D (August 2009). "Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection". Current Opinion in Investigational Drugs. 10 (8): 860–70. PMID 19649930.
- Low JG, Sung C, Wijaya L, Wei Y, Rathore AP, Watanabe S, et al. (August 2014). "Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial". The Lancet. Infectious Diseases. 14 (8): 706–715. doi:10.1016/S1473-3099(14)70730-3. PMID 24877997.