Clay
Clay is a type of fine-grained natural soil material containing clay minerals.[1] Clays develop plasticity when wet, due to a molecular film of water surrounding the clay particles, but become hard, brittle and non–plastic upon drying or firing.[2][3][4] Most pure clay minerals are white or light-coloured, but natural clays show a variety of colours from impurities, such as a reddish or brownish colour from small amounts of iron oxide.[5][6]

Clay is the oldest known ceramic material. Prehistoric humans discovered the useful properties of clay and used it for making pottery. Some of the earliest pottery shards have been dated to around 14,000 BC,[7] and clay tablets were the first known writing medium.[8] Clay is used in many modern industrial processes, such as paper making, cement production, and chemical filtering. Between one-half and two-thirds of the world's population still live or work in buildings made with clay, often baked into brick, as an essential part of its load-bearing structure.
Clay is a very common substance. Shale, formed largely from clay, is the most common sedimentary rock.[9] Although many naturally occurring deposits include both silts and clay, clays are distinguished from other fine-grained soils by differences in size and mineralogy. Silts, which are fine-grained soils that do not include clay minerals, tend to have larger particle sizes than clays. Mixtures of sand, silt and less than 40% clay are called loam. Soils high in swelling clays, which are clay minerals that readily expand in volume when they absorb water, are a major challenge in civil engineering.[1]

Properties
The defining mechanical property of clay is its plasticity when wet and its ability to harden when dried or fired. Clays show a broad range of water content within which they are highly plastic, from a minimum water content (called the plasticity limit) where the clay is just moist enough to mould, to a maximum water content (called the liquid limit) where the moulded clay is just dry enough to hold its shape.[10] The plastic limit of kaolinite clay ranges from about 36% to 40% and its liquid limit ranges from about 58% to 72%.[11] High-quality clay is also tough, as measured by the amount of mechanical work required to roll a sample of clay flat. Its toughness reflects a high degree of internal cohesion.[10]
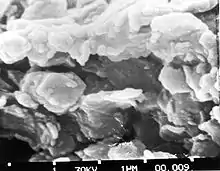
Clay has a high content of clay minerals that give it its plasticity. Clay minerals are hydrous aluminium phyllosilicate minerals, composed of aluminium and silicon ions bonded into tiny, thin plates by interconnecting oxygen and hydroxyl ions. These plates are tough but flexible, and in moist clay, they adhere to each other. The resulting aggregates give clay the cohesion that makes it plastic.[12] In kaolinite clay, the bonding between plates is provided by a film of water molecules that hydrogen bond the plates together. The bonds are weak enough to allow the plates to slip past each other when the clay is being moulded, but strong enough to hold the plates in place and allow the moulded clay to retain its shape after it is moulded. When the clay is dried, most of the water molecules are removed, and the plates hydrogen bond directly to each other, so that the dried clay is rigid but still fragile. If the clay is moistened again, it will once more become plastic. When the clay is fired to the earthenware stage, a dehydration reaction removes additional water from the clay, causing clay plates to irreversibly adhere to each other via stronger covalent bonding, which strengthens the material. The clay mineral, kaolin, is transformed into a non-clay material, metakaolin, which remains rigid and hard if moistened again. Further firing through the stoneware and porcelain stages further recrystallizes the metakaolin into yet stronger minerals such as mullite.[4]
The tiny size and plate form of clay particles gives clay minerals a high surface area. In some clay minerals, the plates carry a negative electrical charge that is balanced by a surrounding layer of positive ions (cations), such as sodium, potassium, or calcium. If the clay is mixed with a solution containing other cations, these can swap places with the cations in the layer around the clay particles, which gives clays a high capacity for ion exchange.[12] The chemistry of clay minerals, including their capacity to retain nutrient cations such as potassium and ammonium, is important to soil fertility.[13]
Clay is a common component of sedimentary rock. Shale is formed largely from clay and is the most common of sedimentary rocks.[9] However, most clay deposits are impure. Many naturally occurring deposits include both silts and clay. Clays are distinguished from other fine-grained soils by differences in size and mineralogy. Silts, which are fine-grained soils that do not include clay minerals, tend to have larger particle sizes than clays. There is, however, some overlap in particle size and other physical properties. The distinction between silt and clay varies by discipline. Geologists and soil scientists usually consider the separation to occur at a particle size of 2 μm (clays being finer than silts), sedimentologists often use 4–5 μm, and colloid chemists use 1 μm.[2] Geotechnical engineers distinguish between silts and clays based on the plasticity properties of the soil, as measured by the soils' Atterberg limits. ISO 14688 grades clay particles as being smaller than 2 μm and silt particles as being larger. Mixtures of sand, silt and less than 40% clay are called loam.
Some clay minerals (such as smectite) are described as swelling clay minerals, because they have a great capacity to take up water, and they increase greatly in volume when they do so. When dried, they shrink back to their original volume. This produces distinctive textures, such as mudcracks or "popcorn" texture, in clay deposits. Soils containing swelling clay minerals (such as bentonite) pose a considerable challenge for civil engineering, because swelling clay can break foundations of buildings and ruin road beds.[1]
Formation

Clay minerals most commonly form by prolonged chemical weathering of silicate-bearing rocks. They can also form locally from hydrothermal activity.[14] Chemical weathering takes place largely by acid hydrolysis due to low concentrations of carbonic acid, dissolved in rainwater or released by plant roots. The acid breaks bonds between aluminium and oxygen, releasing other metal ions and silica (as a gel of orthosilicic acid).)[15]
The clay minerals formed depend on the composition of the source rock and the climate. Acid weathering of feldspar-rich rock, such as granite, in warm climates tends to produce kaolin. Weathering of the same kind of rock under alkaline conditions produces illite. Smectite forms by weathering of igneous rock under alkaline conditions, while gibbsite forms by intense weathering of other clay minerals.[16]
There are two types of clay deposits: primary and secondary. Primary clays form as residual deposits in soil and remain at the site of formation. Secondary clays are clays that have been transported from their original location by water erosion and deposited in a new sedimentary deposit.[17] Secondary clay deposits are typically associated with very low energy depositional environments such as large lakes and marine basins.[14]
Varieties
The main groups of clays include kaolinite, montmorillonite-smectite, and illite. Chlorite, vermiculite,[18] talc, and pyrophyllite[19] are sometimes also classified as clay minerals. There are approximately 30 different types of "pure" clays in these categories, but most "natural" clay deposits are mixtures of these different types, along with other weathered minerals.[20] Clay minerals in clays are most easily identified using X-ray diffraction rather than chemical or physical tests.[21]
Varve (or varved clay) is clay with visible annual layers that are formed by seasonal deposition of those layers and are marked by differences in erosion and organic content. This type of deposit is common in former glacial lakes. When fine sediments are delivered into the calm waters of these glacial lake basins away from the shoreline, they settle to the lake bed. The resulting seasonal layering is preserved in an even distribution of clay sediment banding.[14]
Quick clay is a unique type of marine clay indigenous to the glaciated terrains of Norway, Canada, Northern Ireland, and Sweden.[22] It is a highly sensitive clay, prone to liquefaction, and has been involved in several deadly landslides.[23]
Historical and modern uses

.JPG.webp)
Clays are used for making pottery, both utilitarian and decorative, and construction products, such as bricks, walls, and floor tiles. Different types of clay, when used with different minerals and firing conditions, are used to produce earthenware, stoneware, and porcelain. Prehistoric humans discovered the useful properties of clay. Some of the earliest pottery shards recovered are from central Honshu, Japan. They are associated with the Jōmon culture, and recovered deposits have been dated to around 14,000 BC.[7] Cooking pots, art objects, dishware, smoking pipes, and even musical instruments such as the ocarina can all be shaped from clay before being fired.
Clay tablets were the first known writing medium.[8] Scribes wrote by inscribing them with cuneiform script using a blunt reed called a stylus. Purpose-made clay balls were used as sling ammunition.[24] Clay is used in many industrial processes, such as paper making, cement production, and chemical filtering.[25] Bentonite clay is widely used as a mold binder in the manufacture of sand castings.[26][27]
Clay, being relatively impermeable to water, is also used where natural seals are needed, such as in the cores of dams, or as a barrier in landfills against toxic seepage (lining the landfill, preferably in combination with geotextiles).[28] Studies in the early 21st century have investigated clay's absorption capacities in various applications, such as the removal of heavy metals from waste water and air purification.[29][30]
Medical use
Traditional uses of clay as medicine goes back to prehistoric times. An example is Armenian bole, which is used to soothe an upset stomach. Some animals such as parrots and pigs ingest clay for similar reasons.[31] Kaolin clay and attapulgite have been used as anti-diarrheal medicines.[32]
As a building material
.jpg.webp)
Clay as the defining ingredient of loam is one of the oldest building materials on Earth, among other ancient, naturally-occurring geologic materials such as stone and organic materials like wood.[33] Between one-half and two-thirds of the world's population, in both traditional societies as well as developed countries, still live or work in buildings made with clay, often baked into brick, as an essential part of its load-bearing structure. Also a primary ingredient in many natural building techniques, clay is used to create adobe, cob, cordwood, and rammed earth structures and building elements such as wattle and daub, clay plaster, clay render case, clay floors and clay paints and ceramic building material. Clay was used as a mortar in brick chimneys and stone walls where protected from water.

The forecasted mass of clay minerals to be discharged into the tailings of ore processing makes up millions of tons. Worryingly, when macro- and micro-components are found in non-hazardous concentrations, fewer efforts are put into the environmental management of the tailings, though technogenic sediments offer prospects for reuse and valorization beyond their traditional disposal. Saponite is a demonstrative example of the tailings constituent that is often left unfairly mistreated. Electrochemical separation helps to obtain modified saponite-containing products with high smectite-group minerals concentrations, lower mineral particles size, more compact structure, and greater surface area. These characteristics open possibilities for the manufacture of high-quality ceramics and heavy-metal sorbents from saponite-containing products.[35] Furthermore, tail grinding occurs during the preparation of the raw material for ceramics; this waste reprocessing is of high importance for the use of clay pulp as a neutralizing agent, as fine particles are required for the reaction. Experiments on the Histosol deacidification with the alkaline clay slurry demonstrated that neutralization with the average pH level of 7.1 is reached at 30% of the pulp added and an experimental site with perennial grasses proved the efficacy of the technique.[34]
See also
- Argillaceous minerals
- Industrial plasticine – A modeling material which is mainly used by automotive design studios
- Clay animation
- Clay chemistry – The chemical structures, properties and reactions of clay minerals
- Clay court
- Clay panel
- Clay pit
- Geophagia – Practice of eating earth or soil-like substrates such as clay or chalk
- Graham Cairns-Smith
- Expansive clay
- London Clay – Marine geological formation which crops out in the southeast of England.
- Modelling clay
- Paper clay
- Particle size
- Plasticine – Brand of modeling clay
- Vertisol – Clay-rich soil, prone to cracking
- Clay–water interaction – Various progressive interactions between clay minerals and water
Notes
- Olive et al. 1989.
- Guggenheim & Martin 1995, pp. 255–256.
- Science Learning Hub 2010.
- Breuer 2012.
- Klein & Hurlbut 1993, pp. 512-514.
- Nesse 2000, pp. 252-257.
- Scarre 2005, p. 238.
- Ebert 2011, p. 64.
- Boggs 2006, p. 140.
- Moreno-Maroto & Alonso-Azcárate 2018.
- White 1949.
- Bergaya & Lagaly 2006, pp. 1-18.
- Hodges 2010.
- Foley 1999.
- Leeder 2011, pp. 5-11.
- Leeder 2011, pp. 10-11.
- Murray 2002.
- Nesse 2000, p. 253.
- Klein & Hurlbut 1993, pp. 514-515.
- Klein & Hurlbut 1993, p. 512.
- Nesse 2000, p. 256.
- Rankka et al. 2004.
- Natural Resources Canada 2005.
- Forouzan et al. 2012.
- Nesse 2000, p. 257.
- Boylu 2011.
- Eisenhour & Brown 2009.
- Koçkar, Akgün & Aktürk 2005.
- García-Sanchez, Alvarez-Ayuso & Rodriguez-Martin 2002.
- Churchman et al. 2006.
- Diamond 1999.
- Dadu et al. 2015.
- Grim 2016.
- Pashkevich, M.A.; Alekseenko, A.V. (2020). "Reutilization Prospects of Diamond Clay Tailings at the Lomonosov Mine, Northwestern Russia". Minerals. 10 (6): 517. doi:10.3390/min10060517.
 This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license. - Chanturiya, V.A.; Minenko, V.G.; Makarov, D.V. (2018). "Advanced Techniques of Saponite Recovery from Diamond Processing Plant Water and Areas of Saponite Application". Minerals. 8 (12): 549. doi:10.3390/min8120549.
 This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license.
References
- Clay mineral nomenclature American Mineralogist.
- Bergaya, F.; Lagaly, G. (2006). "Chapter 1 General Introduction: Clays, Clay Minerals, and Clay Science". Developments in Clay Science. 1: 1–18. doi:10.1016/S1572-4352(05)01001-9. ISBN 9780080441832.
- Boggs, Sam (2006). Principles of sedimentology and stratigraphy (4th ed.). Upper Saddle River, N.J.: Pearson Prentice Hall. ISBN 0131547283.
- Boylu, Feridun (1 April 2011). "Optimization of foundry sand characteristics of soda-activated calcium bentonite". Applied Clay Science. 52 (1): 104–108. doi:10.1016/j.clay.2011.02.005.
- Breuer, Stephen (July 2012). "The chemistry of pottery" (PDF). Education in Chemistry: 17–20. Retrieved 8 December 2020.
- Churchman, G. J.; Gates, W. P.; Theng, B. K. G.; Yuan, G. (2006). Faïza Bergaya, Benny K. G. Theng and Gerhard Lagaly (ed.). "Chapter 11.1 Clays and Clay Minerals for Pollution Control". Developments in Clay Science. Handbook of Clay Science. Elsevier. 1: 625–675. doi:10.1016/S1572-4352(05)01020-2. ISBN 9780080441832.
- Dadu, Ramona; Hu, Mimi I.; Cleeland, Charles; Busaidy, Naifa L.; Habra, Mouhammed; Waguespack, Steven G.; Sherman, Steven I.; Ying, Anita; Fox, Patricia; Cabanillas, Maria E. (October 2015). "Efficacy of the Natural Clay, Calcium Aluminosilicate Anti-Diarrheal, in Reducing Medullary Thyroid Cancer–Related Diarrhea and Its Effects on Quality of Life: A Pilot Study". Thyroid. 25 (10): 1085–1090. doi:10.1089/thy.2015.0166. PMC 4589264. PMID 26200040.
- Diamond, Jared M. (1999). "Diamond on Geophagy". ucla.edu. Archived from the original on 28 May 2015.
- Ebert, John David (31 August 2011). The New Media Invasion: Digital Technologies and the World They Unmake. McFarland. ISBN 9780786488186. Archived from the original on 24 December 2017.
- Ehlers, Ernest G. and Blatt, Harvey (1982). 'Petrology, Igneous, Sedimentary, and Metamorphic' San Francisco: W.H. Freeman and Company. ISBN 0-7167-1279-2.
- Eisenhour, D. D.; Brown, R. K. (1 April 2009). "Bentonite and Its Impact on Modern Life". Elements. 5 (2): 83–88. doi:10.2113/gselements.5.2.83.
- Foley, Nora K. (September 1999). "Environmental Characteristics of Clays and Clay Mineral Deposits". usgs.gov. Archived from the original on 8 December 2008.
- Forouzan, Firoozeh; Glover, Jeffrey B.; Williams, Frank; Deocampo, Daniel (1 December 2012). "Portable XRF analysis of zoomorphic figurines, "tokens," and sling bullets from Chogha Gavaneh, Iran". Journal of Archaeological Science. 39 (12): 3534–3541. doi:10.1016/j.jas.2012.04.010.
- García-Sanchez, A.; Alvarez-Ayuso, E.; Rodriguez-Martin, F. (1 March 2002). "Sorption of As(V) by some oxyhydroxides and clay minerals. Application to its immobilization in two polluted mining soils". Clay Minerals. 37 (1): 187–194. Bibcode:2002ClMin..37..187G. doi:10.1180/0009855023710027. S2CID 101864343.
- Grim, Ralph (2016). "Clay mineral". Encyclopædia Britannica. Archived from the original on 9 December 2015. Retrieved 10 January 2016.
- Guggenheim, Stephen; Martin, R. T. (1995), "Definition of clay and clay mineral: Journal report of the AIPEA nomenclature and CMS nomenclature committees", Clays and Clay Minerals, 43 (2): 255–256, Bibcode:1995CCM....43..255G, doi:10.1346/CCMN.1995.0430213
- Hillier S. (2003) "Clay Mineralogy." pp 139–142 In Middleton G.V., Church M.J., Coniglio M., Hardie L.A. and Longstaffe F.J. (Editors) Encyclopedia of Sediments and Sedimentary Rocks. Kluwer Academic Publishers, Dordrecht.
- Hodges, S.C. (2010). "Soil fertility basics" (PDF). Soil Science Extension, North Carolina State University. Retrieved 8 December 2020.
- Klein, Cornelis; Hurlbut, Cornelius S., Jr. (1993). Manual of mineralogy : (after James D. Dana) (21st ed.). New York: Wiley. ISBN 047157452X.
- Koçkar, Mustafa K.; Akgün, Haluk; Aktürk, Özgür (November 2005). "Preliminary evaluation of a compacted bentonite / sand mixture as a landfill liner material (Abstract)]". Department of Geological Engineering, Middle East Technical University, Ankara, Turkey. Archived from the original on 4 December 2008.
- Leeder, M. R. (2011). Sedimentology and sedimentary basins : from turbulence to tectonics (2nd ed.). Chichester, West Sussex, UK: Wiley-Blackwell. ISBN 978-1-40517783-2.
- Moreno-Maroto, José Manuel; Alonso-Azcárate, Jacinto (September 2018). "What is clay? A new definition of "clay" based on plasticity and its impact on the most widespread soil classification systems". Applied Clay Science. 161: 57–63. doi:10.1016/j.clay.2018.04.011.
- Murray, H. (2002). "Industrial clays case study" (PDF). Mining, Minerals and Sustainable Development. 64: 1–9. Retrieved 8 December 2020.
- "Landslides". Geoscape Ottawa-Gatineau. Natural Resources Canada. 7 March 2005. Archived from the original on 24 October 2005. Retrieved 21 July 2016.
- Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. ISBN 9780195106916.
- Olive, W.W.; Chleborad, A.F.; Frahme, C.W.; Shlocker, Julius; Schneider, R.R.; Schuster, R.L. (1989). "Swelling Clays Map of the Conterminous United States". U.S. Geological Survey Miscellaneous Investigations Series Map. I-1940. Retrieved 8 December 2020.
- Rankka, Karin; Andersson-Sköld, Yvonne; Hultén, Carina; Larsson, Rolf; Leroux, Virginie; Dahlin, Torleif (2004). "Quick clay in Sweden" (PDF). Report No. 65. Swedish Geotechnical Institute. Archived from the original (PDF) on 4 April 2005. Retrieved 20 April 2005.
- Scarre, C. (2005). The Human Past. London: Thames and Hudson. ISBN 0500290636.
- "What is clay". Science Learning Hub. University of Waikato. Archived from the original on 3 January 2016. Retrieved 10 January 2016.
- White, W.A. (1949). "Atterberg plastic limits of clay minerals" (PDF). American Mineralogist: Journal of Earth and Planetary Materials. 34 (7–8): 508–512. Retrieved 7 December 2020.
External links
| Look up clay in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Clay. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Clay. |
| Wikiquote has quotations related to: Clay |