Conductivity (electrolytic)
Conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is Siemens per meter (S/m).
Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution.[1] For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems.
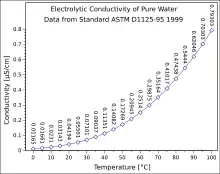
In many cases, conductivity is linked directly to the total dissolved solids (T.D.S.). High quality deionized water has a conductivity of about 0.5 μS/cm at 25 °C, typical drinking water is in the range of 200 - 800 μS/cm, while sea water is about 50 mS/cm[2] (or 50,000 μS/cm). Conductivity is traditionally determined by connecting the electrolyte in a Wheatstone bridge. Dilute solutions follow Kohlrausch's Laws of concentration dependence and additivity of ionic contributions. Lars Onsager gave a theoretical explanation of Kohlrausch's law by extending Debye–Hückel theory.
Units
The SI unit of conductivity is S/m and, unless otherwise qualified, it refers to 25 °C. More generally encountered is the traditional unit of μS/cm.
The commonly used standard cell has a width of 1 cm, and thus for very pure water in equilibrium with air would have a resistance of about 106 ohms, known as a megohm. Ultra-pure water could achieve 18 megohms or more. Thus in the past, megohm-cm was used, sometimes abbreviated to "megohm". Sometimes, conductivity is given in "microsiemens" (omitting the distance term in the unit). While this is an error, it can often be assumed to be equal to the traditional μS/cm.
The conversion of conductivity to the total dissolved solids depends on the chemical composition of the sample and can vary between 0.54 and 0.96. Typically, the conversion is done assuming that the solid is sodium chloride, i.e., 1 μS/cm is then equivalent to about 0.64 mg of NaCl per kg of water.
Molar conductivity has the SI unit S m2 mol−1. Older publications use the unit Ω−1 cm2 mol−1.
Measurement

The electrical conductivity of a solution of an electrolyte is measured by determining the resistance of the solution between two flat or cylindrical electrodes separated by a fixed distance.[3] An alternating voltage is used in order to avoid electrolysis. The resistance is measured by a conductivity meter. Typical frequencies used are in the range 1–3 kHz. The dependence on the frequency is usually small,[4] but may become appreciable at very high frequencies, an effect known as the Debye–Falkenhagen effect.
A wide variety of instrumentation is commercially available.[5] Most commonly, two types of electrode sensors are used, electrode-based sensors and inductive sensors. Electrode sensors with a static design are suitable for low and moderate conductivities, and exist in various types, having either 2 or 4 electrodes, where electrodes can be arrange oppositely, flat or in a cylinder.[6] Electrode cells with a flexible design, where the distance between two oppositely arranged electrodes can be varied, offer high accuracy and can also be used for the measurement of highly conductive media.[7] Inductive sensors are suitable for harsh chemical conditions but require larger sample volumes than electrode sensors.[8] Conductivity sensors are typically calibrated with KCl solutions of known conductivity. Electrolytic conductivity is highly temperature dependent but many commercial systems offer automatic temperature correction. Tables of reference conductivities are available for many common solutions.[9]
Definitions
Resistance, R, is proportional to the distance, l, between the electrodes and is inversely proportional to the cross-sectional area of the sample, A (noted S on the Figure above). Writing ρ (rho) for the specific resistance (or resistivity),
In practice the conductivity cell is calibrated by using solutions of known specific resistance, ρ*, so the quantities l and A need not be known precisely.[10] If the resistance of the calibration solution is R*, a cell-constant, C, is derived.
The specific conductance (conductivity), κ (kappa) is the reciprocal of the specific resistance.
Conductivity is also temperature-dependent. Sometimes the ratio of l and A is called as the cell constant, denoted as G*, and conductance is denoted as G. Then the specific conductance κ (kappa), can be more conveniently written as
Theory
The specific conductance of a solution containing one electrolyte depends on the concentration of the electrolyte. Therefore, it is convenient to divide the specific conductance by concentration. This quotient, termed molar conductivity, is denoted by Λm
Strong electrolytes
Strong electrolytes are hypothesized to dissociate completely in solution. The conductivity of a solution of a strong electrolyte at low concentration follows Kohlrausch's Law
where is known as the limiting molar conductivity, K is an empirical constant and c is the electrolyte concentration. (Limiting here means "at the limit of the infinite dilution".) In effect, the observed conductivity of a strong electrolyte becomes directly proportional to concentration, at sufficiently low concentrations i.e. when
As the concentration is increased however, the conductivity no longer rises in proportion. Moreover, Kohlrausch also found that the limiting conductivity of an electrolyte;
- and are the limiting molar conductivities of the individual ions.
The following table gives values for the limiting molar conductivities for some selected ions.[11]
| Table of limiting ion conductivity in water at 298 K (approx. 25 °C)[11] | |||||||
|---|---|---|---|---|---|---|---|
| Cations | +o / mSm2mol−1 | Cations | +o / mSm2mol−1 | Anions | −o / mSm2mol−1 | Anions | −o / mSm2mol−1 |
| H+ | 34.982 | Ba2+ | 12.728 | −OH | 19.8 | SO42− | 15.96 |
| Li+ | 3.869 | Mg2+ | 10.612 | Cl− | 7.634 | C2O42− | 7.4 |
| Na+ | 5.011 | La3+ | 20.88 | Br− | 7.84 | HC2O4− | 40.2 ??? |
| K+ | 7.352 | Rb+ | 7.64 | I− | 7.68 | HCOO− | 5.6 |
| NH4+ | 7.34 | Cs+ | 7.68 | NO3− | 7.144 | CO32− | 7.2 |
| Ag+ | 6.192 | Be2+ | 4.50 | CH3COO− | 4.09 | HSO32− | 5.0 |
| Ca2+ | 11.90 | ClO4− | 6.80 | SO32− | 7.2 | ||
| Co(NH3)63+ | 10.2 | F− | 5.50 | ||||
An interpretation of these results was based on the theory of Debye and Hückel, yielding the Debye-Hückel-Onsager theory:[12]
where A and B are constants that depend only on known quantities such as temperature, the charges on the ions and the dielectric constant and viscosity of the solvent. As the name suggests, this is an extension of the Debye–Hückel theory, due to Onsager. It is very successful for solutions at low concentration.
Weak electrolytes
A weak electrolyte is one that is never fully dissociated (i.e. there are a mixture of ions and complete molecules in equilibrium). In this case there is no limit of dilution below which the relationship between conductivity and concentration becomes linear. Instead, the solution becomes ever more fully dissociated at weaker concentrations, and for low concentrations of "well behaved" weak electrolytes, the degree of dissociation of the weak electrolyte becomes proportional to the inverse square root of the concentration.
Typical weak electrolytes are weak acids and weak bases. The concentration of ions in a solution of a weak electrolyte is less than the concentration of the electrolyte itself. For acids and bases the concentrations can be calculated when the value(s) of the acid dissociation constant(s) is (are) known.
For a monoprotic acid, HA, obeying the inverse square root law, with a dissociation constant Ka, an explicit expression for the conductivity as a function of concentration, c, known as Ostwald's dilution law, can be obtained.
Various solvents exhibit the same dissociation if the ratio of relative permittivities equals the ratio cubic roots of concentrations of the electrolytes (Walden's rule).
Higher concentrations
Both Kohlrausch's law and the Debye-Hückel-Onsager equation break down as the concentration of the electrolyte increases above a certain value. The reason for this is that as concentration increases the average distance between cation and anion decreases, so that there is more inter-ionic interaction. Whether this constitutes ion association is a moot point. However, it has often been assumed that cation and anion interact to form an ion pair. Thus the electrolyte is treated as if it were like a weak acid and a constant, K, can be derived for the equilibrium
- A+ + B− ⇌ A+B−; K=[A+] [B−]/[A+B−]
Davies describes the results of such calculations in great detail, but states that K should not necessarily be thought of as a true equilibrium constant, rather, the inclusion of an "ion-association" term is useful in extending the range of good agreement between theory and experimental conductivity data.[13] Various attempts have been made to extend Onsager's treatment to more concentrated solutions.[14]
The existence of a so-called conductance minimum in solvents having the relative permittivity under 60 has proved to be a controversial subject as regards interpretation. Fuoss and Kraus suggested that it is caused by the formation of ion triplets,[15] and this suggestion has received some support recently.[16][17]
Other developments on this topic have been done by Theodore Shedlovsky,[18] E. Pitts,[19] R. M. Fuoss,[20][21] Fuoss and Shedlovsky,[22] Fuoss and Onsager.[23][24]
Mixed solvents systems
The limiting equivalent conductivity of solutions based on mixed solvents like water alcohol has minima depending on the nature of alcohol. For methanol the minimum is at 15 molar % water,[18][25][26] and for the ethanol at 6 molar % water.[27]
Conductivity versus temperature
Generally the conductivity of a solution increases with temperature, as the mobility of the ions increases. For comparison purposes reference values are reported at an agreed temperature, usually 298 K (≈ 25 °C), although occasionally 20 °C is used. So called 'compensated' measurements are made at a convenient temperature but the value reported is a calculated value of the expected value of conductivity of the solution, as if it had been measured at the reference temperature. Basic compensation is normally done by assuming a linear increase of conductivity versus temperature of typically 2% per Kelvin. This value is broadly applicable for most salts at room temperature. Determination of the precise temperature coefficient for a specific solution is simple and instruments are typically capable of applying the derived coefficient (i.e. other than 2%).
Solvent isotopic effect
The change in conductivity due to the isotope effect for deuterated electrolytes is sizable.[28]
Applications
Notwithstanding the difficulty of theoretical interpretation, measured conductivity is a good indicator of the presence or absence of conductive ions in solution, and measurements are used extensively in many industries.[29] For example, conductivity measurements are used to monitor quality in public water supplies, in hospitals, in boiler water and industries which depend on water quality such as brewing. This type of measurement is not ion-specific; it can sometimes be used to determine the amount of total dissolved solids (T.D.S.) if the composition of the solution and its conductivity behavior are known.[1] Conductivity measurements made to determine water purity will not respond to non conductive contaminants (many organic compounds fall into this category), therefore additional purity tests may be required depending on application.
Sometimes, conductivity measurements are linked with other methods to increase the sensitivity of detection of specific types of ions. For example, in the boiler water technology, the boiler blowdown is continuously monitored for "cation conductivity", which is the conductivity of the water after it has been passed through a cation exchange resin. This is a sensitive method of monitoring anion impurities in the boiler water in the presence of excess cations (those of the alkalizing agent usually used for water treatment). The sensitivity of this method relies on the high mobility of H+ in comparison with the mobility of other cations or anions. Beyond cation conductivity, there are analytical instruments designed to measure Degas conductivity, where conductivity is measured after dissolved carbon dioxide has been removed from the sample, either through reboiling or dynamic degassing.
Conductivity detectors are commonly used with ion chromatography.[30]
See also
- Einstein relation (kinetic theory)
- Born equation
- Debye–Falkenhagen effect
- Law of dilution
- Ion transport number
- Ionic atmosphere
- Wien effect
- Svante Arrhenius
- Alfred Werner - coordination chemistry
- Conductimetric titration - methods to determine the equivalence point
References
- Gray, James R. (2004). "Conductivity Analyzers and Their Application". In Down, R. D.; Lehr, J. H. (eds.). Environmental Instrumentation and Analysis Handbook. Wiley. pp. 491–510. ISBN 978-0-471-46354-2. Retrieved 10 May 2009.
- "Water Conductivity". Lenntech. Retrieved 5 January 2013.
- Bockris, J. O'M.; Reddy, A.K.N; Gamboa-Aldeco, M. (1998). Modern Electrochemistry (2nd. ed.). Springer. ISBN 0-306-45555-2. Retrieved 10 May 2009.
- Marija Bešter-Rogač and Dušan Habe, "Modern Advances in Electrical Conductivity Measurements of Solutions", Acta Chim. Slov. 2006, 53, 391–395 (pdf)
- Boyes, W. (2002). Instrumentation Reference Book (3rd. ed.). Butterworth-Heinemann. ISBN 0-7506-7123-8. Retrieved 10 May 2009.
- Gray, p 495
- Doppelhammer, Nikolaus; Pellens, Nick; Martens, Johan; Kirschhock, Christine E. A.; Jakoby, Bernhard; Reichel, Erwin K. (27 October 2020). "Moving Electrode Impedance Spectroscopy for Accurate Conductivity Measurements of Corrosive Ionic Media". ACS Sensors. 5 (11): 3392–3397. doi:10.1021/acssensors.0c01465. PMC 7706010. PMID 33107724.
- Ghosh, Arun K. (2013). Introduction to measurements and instrumentation (4th ed., Eastern economy ed.). Delhi: PH Learning. ISBN 978-81-203-4625-3. OCLC 900392417.
- "Conductivity ordering guide" (PDF). EXW Foxboro. 3 October 1999. Archived from the original (PDF) on 7 September 2012. Retrieved 5 January 2013.
- "ASTM D1125 - 95(2005) Standard Test Methods for Electrical Conductivity and Resistivity of Water". Retrieved 12 May 2009.
- Adamson, Arthur W. (1973). Textbook of Physical Chemistry. London: Academic Press inc. p. 512.
- Wright, M.R. (2007). An Introduction to Aqueous Electrolyte Solutions. Wiley. ISBN 978-0-470-84293-5.
- Davies, C. W. (1962). Ion Association. London: Butterworths.
- Miyoshi, K. (1973). "Comparison of the Conductance Equations of Fuoss–Onsager, Fuoss–Hsia and Pitts with the Data of Bis(2,9-dimethyl-1,10-phenanthroline)Cu(I) Perchlorate". Bull. Chem. Soc. Jpn. 46 (2): 426–430. doi:10.1246/bcsj.46.426.
- Fuoss, R. M.; Kraus, C. A. (1935). "Properties of Electrolytic Solutions. XV. Thermodynamic Properties of Very Weak Electrolytes". J. Am. Chem. Soc. 57: 1–4. doi:10.1021/ja01304a001.
- Weingärtner, H.; Weiss, V. C.; Schröer, W. (2000). "Ion association and electrical conductance minimum in Debye–Hückel-based theories of the hard sphere ionic fluid". J. Chem. Phys. 113 (2): 762–. Bibcode:2000JChPh.113..762W. doi:10.1063/1.481822.
- Schröer, W.; Weingärtner, H. (2004). "Structure and criticality of ionic fluids". Pure Appl. Chem. 76 (1): 19–27. doi:10.1351/pac200476010019. S2CID 39716065. pdf
- Shedlovsky, Theodore (1932). "The Electrolytic Conductivity of some Uni-Univalent Electrolytes in Water at 25°". Journal of the American Chemical Society. American Chemical Society (ACS). 54 (4): 1411–1428. doi:10.1021/ja01343a020. ISSN 0002-7863.
- Pitts, E.; Coulson, Charles Alfred (1953). "An extension of the theory of the conductivity and viscosity of electrolyte solutions". Proc. Roy. Soc. A217 (1128): 43. Bibcode:1953RSPSA.217...43P. doi:10.1098/rspa.1953.0045. S2CID 123363978.
- Fuoss, Raymond M. (1958). "Conductance of Ionophores". Journal of the American Chemical Society. American Chemical Society (ACS). 80 (12): 3163. doi:10.1021/ja01545a064. ISSN 0002-7863.
- Fuoss, Raymond M. (1959). "Conductance of Dilute Solutions of 1-1 Electrolytes1". Journal of the American Chemical Society. American Chemical Society (ACS). 81 (11): 2659–2662. doi:10.1021/ja01520a016. ISSN 0002-7863.
- Fuoss, Raymond M.; Shedlovsky, Theodore. (1949). "Extrapolation of Conductance Data for Weak Electrolytes". Journal of the American Chemical Society. American Chemical Society (ACS). 71 (4): 1496–1498. doi:10.1021/ja01172a507. ISSN 0002-7863.
- Fuoss, Raymond M.; Onsager, Lars (1964). "The Conductance of Symmetrical Electrolytes.1aIV. Hydrodynamic and Osmotic Terms in the Relaxation Field". The Journal of Physical Chemistry. American Chemical Society (ACS). 68 (1): 1–8. doi:10.1021/j100783a001. ISSN 0022-3654.
- Fuoss, Raymond M.; Onsager, Lars; Skinner, James F. (1965). "The Conductance of Symmetrical Electrolytes. V. The Conductance Equation1,2". The Journal of Physical Chemistry. American Chemical Society (ACS). 69 (8): 2581–2594. doi:10.1021/j100892a017. ISSN 0022-3654.
- Shedlovsky, Theodore; Kay, Robert L. (1956). "The Ionization Constant of Acetic Acid in Water–Methanol Mixtures at 25° from Conductance Measurements". The Journal of Physical Chemistry. American Chemical Society (ACS). 60 (2): 151–155. doi:10.1021/j150536a003. ISSN 0022-3654.
- Strehlow, H. (1960). "Der Einfluß von Wasser auf die Äquivalentleitfähigkeit von HCl in Methanol". Zeitschrift für Physikalische Chemie. Walter de Gruyter GmbH. 24 (3_4): 240–248. doi:10.1524/zpch.1960.24.3_4.240. ISSN 0942-9352.
- Bezman, Irving I.; Verhoek, Frank H. (1945). "The Conductance of Hydrogen Chloride and Ammonium Chloride in Ethanol-Water Mixtures". Journal of the American Chemical Society. American Chemical Society (ACS). 67 (8): 1330–1334. doi:10.1021/ja01224a035. ISSN 0002-7863.
- Biswas, Ranjit (1997). "Limiting Ionic Conductance of Symmetrical, Rigid Ions in Aqueous Solutions: Temperature Dependence and Solvent Isotope Effects". Journal of the American Chemical Society. 119 (25): 5946–5953. doi:10.1021/ja970118o.
- "Electrolytic conductivity measurement, Theory and practice" (PDF). Aquarius Technologies Pty Ltd. Archived from the original (PDF) on 12 September 2009.
- "Detectors for ion-exchange chromatography". Retrieved 17 May 2009.
Further reading
| Wikimedia Commons has media related to Conductometry. |
- Hans Falkenhagen, Theorie der Elektrolyte, S. Hirzel Verlag, Leipzig, 1971
- Friedman, Harold L. (1965). "Relaxation Term of the Limiting Law of the Conductance of Electrolyte Mixtures". The Journal of Chemical Physics. 42 (2): 462–469. Bibcode:1965JChPh..42..462F. doi:10.1063/1.1695956.
- Conductivity of concentrated solutions of electrolytes in methyl and ethyl alcohols
- Concentrated solutions and ionic cloud model
- H. L. Friedman, F. Franks, Aqueous Simple Electrolytes Solutions