Conjugated fatty acid
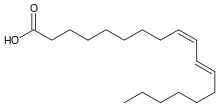
Conjugated fatty acids are polyunsaturated fatty acids in which at least one pair of double bonds is separated by only one single bond.[1] The conjugation implies four consecutive unsaturated links (i.e., CH rather than CH2 groups), and results in a delocalization of electrons along the double-bonded carbons. An example of a conjugated fatty acid is the rumenic acid, found in the meat and milk of ruminants.
Some conjugated fatty acids may confer health benefits ranging from the prevention of hypertension to protection against certain forms of cancer, although more research is needed to confirm such effects.[2]
Conjugated fatty acids have different groups, and one of them is the conjugated linoleic acid isomers.[3] Conjugated linoleic acids are the most investigated conjugated fatty acids.[4] The reason why the isomer of conjugated fatty acids are studied is because they have the potential to treat human illnesses.[3] A biological activity that conjugated fatty acids have been studied for is the reduction of body fat and the increasing of lean body mass.[5] There are experimental models that proved the ability of conjugated linoleic acid to reduce fat levels.[6] This is why the scientific community see the potential to use conjugated fatty acids with active treatments to prevent obesity.[5] However, research studies made in humans are in disagreement even though these studies have proven that the supplementation of conjugated linoleic acid has some benefits such as weight loss.[6]
Conjugated fatty acids can have either a cis or trans configuration. For instance, the rumenic acid (cis9, trans11) is a conjugated trans fatty acid.
See also
References
- Rawat, Richa; Yu, Xiao-Hong; Sweet, Marie; Shanklin, John (26 March 2012). "Conjugated Fatty Acid Synthesis". Journal of Biological Chemistry. 287 (20): 16230–16237. doi:10.1074/jbc.M111.325316. PMC 3351362. PMID 22451660.
- Nagao, K; Yanagita, T (2005). "Conjugated fatty acids in food and their health benefits". Journal of Bioscience and Bioengineering. 100 (2): 152–7. doi:10.1263/jbb.100.152. PMID 16198256.
- Hennessy, Alan A.; Ross, Paul R.; Fitzgerald, Gerald F.; Stanton, Catherine (April 2016). "Sources and Bioactive Properties of Conjugated Dietary Fatty Acids". Lipids. 51 (4): 377–397. doi:10.1007/s11745-016-4135-z. ISSN 0024-4201. PMID 26968402. S2CID 3941392.
- Gong, Mengyue; Hu, Yulin; Wei, Wei; Jin, Qingzhe; Wang, Xingguo (2019-12-01). "Production of conjugated fatty acids: A review of recent advances". Biotechnology Advances. 37 (8): 107454. doi:10.1016/j.biotechadv.2019.107454. ISSN 0734-9750. PMID 31639444.
- Park, Yeonhwa; Park, Yooheon (January 2012), Patil, Bhimanagouda S.; Jayaprakasha, Guddadarangavvanahally K.; Murthy, Kotamballi N. Chidambara; Seeram, Navindra P. (eds.), "Conjugated Fatty Acids as a Prevention Tool for Obesity and Osteoporosis", Emerging Trends in Dietary Components for Preventing and Combating Disease, American Chemical Society, 1093, pp. 393–405, doi:10.1021/bk-2012-1093.ch022, ISBN 978-0-8412-2664-7
- Lehnen, Tatiana Ederich; da Silva, Marcondes Ramos; Camacho, Augusto; Marcadenti, Aline; Lehnen, Alexandre Machado (December 2015). "A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism". Journal of the International Society of Sports Nutrition. 12 (1): 36. doi:10.1186/s12970-015-0097-4. ISSN 1550-2783. PMC 4574006. PMID 26388708.