Corey-Nicolaou macrolactonization
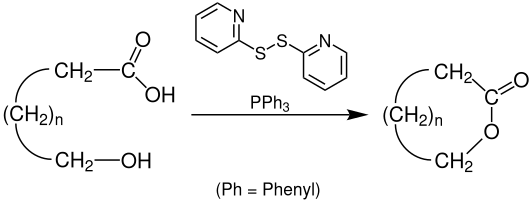
Corey-Nicolaou macrolactonization is a named reaction of organic chemistry, for the synthesis of lactones from hydroxy acids, found in 1974.[1] The reaction should take place in a polar aprotic solvent with mild conditions,[2] with the use of 2,2'-Dipyridyldisulfide and triphenylphosphine.
| Corey-Nicolaou macrolactonization | |
|---|---|
| Named after | Elias James Corey K. C. Nicolaou |
| Reaction type | Name reaction |
Mechanism
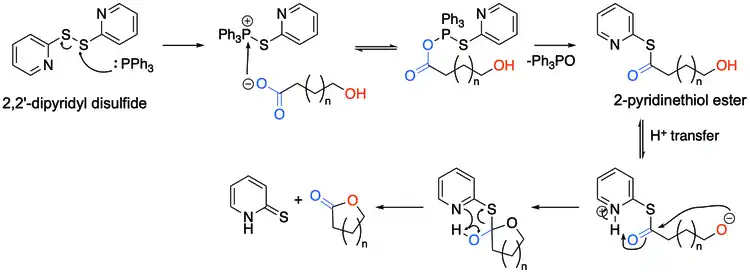
The hydroxy acid first reacts with the 2,2'-Dipyridyldisulfide to form the corresponding 2-pyridinethiol ester, and after a proton transfer, the alkoxide attacks the carbonyl carbon, forming a tetrahedral transition state, before resolving back to the desired lactone and 2-pyridinethione.
See Also
References
- Corey, E.J., Nicolaou, K.C. (1974). "Efficient and mild lactonization method for the synthesis of macrolides". J. Am. Chem. Soc. 96: 5614–5616.CS1 maint: multiple names: authors list (link)
- Meng, Q., Hesse, M. (1992). "Ring-closure methods in the synthesis of macrocyclic natural products". Top. Curr. Chem. 161: 107–176.CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.