Cornforth rearrangement
In organic chemistry, the Cornforth rearrangement is a rearrangement reaction of a 4-acyloxazole in which the group attached to an acyl on position 4 and the substituent on position 5 of an oxazole ring exchange places.[1] It was first reported in 1949, and is named for John Cornforth.
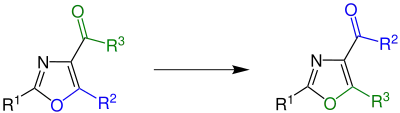 Cornforth rearrangement overall reaction
Cornforth rearrangement overall reaction
Mechanism
The mechanism of the Cornforth rearrangement begins by a thermal pericyclic ring opening which furnishes a nitrile intermediate 1, which then undergoes rearrangement to the oxazole, which is isomeric to the starting compound.
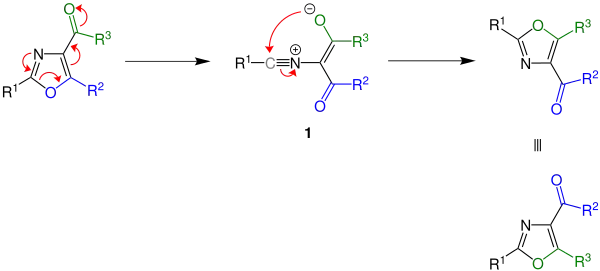
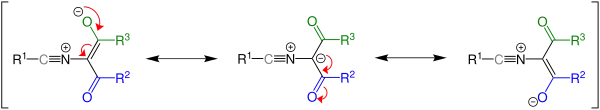
The ylide intermediate has several resonance contributors and the stability of said structures affects the outcome of the reaction, since the intermediate will revert to the starting material if the third resonance structure is most stable. Whether the reaction takes place is dependent on the energy difference between the starting material and the product.
References
- Dewar, Michael J. S. (1974). "Cornforth rearrangement". J. Am. Chem. Soc. 96 (19): 6148–6152. doi:10.1021/ja00826a030.