Cytochalasin E
Cytochalasin E, a member of the cytochalasin group, is an inhibitor of actin polymerization in blood platelets. It inhibits angiogenesis and tumor growth. Unlike cytochalasin A and cytochalasin B, it does not inhibit glucose transport.
 | |
| Names | |
|---|---|
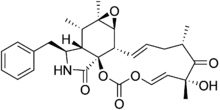
| IUPAC name
(1S,5E,7R,9S,11E,13S,14S,16R,17S,18S,19S)-19-benzyl-7-hydroxy-7,9,16,17-tetramethyl-2,4,15-trioxa-20-azatetracyclo[11.8.0.01,18.014,16]henicosa-5,11-diene-3,8,21-trione | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.048.018 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C28H33NO7 | |
| Molar mass | 495.572 g·mol−1 |
| Density | 1.309 g/ml |
| Hazards | |
| Main hazards | Toxic |
| GHS pictograms |   |
| GHS Signal word | Danger |
| H300, H310, H330, H361 | |
| P201, P202, P260, P262, P264, P270, P271, P280, P281, P284, P301+310, P302+350, P304+340, P308+313, P310, P320, P321, P322, P330, P361, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Because of its antiangiogenic effect, cytochalasin E is a potential drug for age-related macular degeneration, a kind of blindness caused by an abnormal proliferation of blood vessels in the eye.[2]
Cytochalasin E was found to be a potent and selective inhibitor of bovine capillary endothelial (BCE) cell proliferation. Cytochalasin E differs from other cytochalasin molecules by having an epoxide, which is required for specificity and potency. Cytochalasin E is a potent antiangiogenic agent that may be useful for treatments of cancer and other pathologic angiogenesis.[3]
References
- Cytochalasin E from Aspergillus clavatus at Sigma-Aldrich
- eyesight.org Archived 2006-05-19 at the Wayback Machine
- Udagawa, T; Yuan, J; Panigrahy, D; Chang, YH; Shah, J; D'Amato, RJ (August 2000). "Cytochalasin E, an epoxide containing Aspergillus-derived fungal metabolite, inhibits angiogenesis and tumor growth". J. Pharmacol. Exp. Ther. 294: 421–7. PMID 10900214.