DePriester chart
DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature. The original chart was put forth by C.L. DePriester in an article in Chemical Engineering Progress in 1953. These nomograms have two vertical coordinates, one for pressure, and another for temperature. "K" values, representing the tendency of a given chemical species to partition itself preferentially between liquid and vapor phases, are plotted in between.[1][2][3][4] Many DePriester charts have been printed for simple hydrocarbons.[5][6]
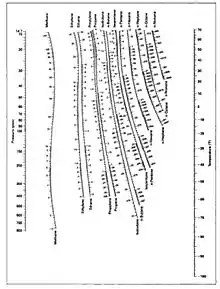
A DePriester Chart
Example
For example, to find the K value of methane at 100 psia and 60 °F.
- On the left-hand vertical axis, locate and mark the point containing the pressure 100 psia.
- On the right-hand vertical axis, locate and mark the point containing the temperature 60°F.
- Connect the points with a straight line.
- Note where the line crosses the methane axis. Read this K-value off the chart (approximately 21.3).
References
- Dambal, Ajey. "DePriester Charts". Thermodynamics - Chemical Engineering (Washington University).
- "Pbub and Pdew calculation based on the DePriester chart for a fast pyrolysis and hydrotreating process" (PDF). Biofuelsacademy.org.
- McKetta Jr., John J. (24 September 1992). Unit Operations Handbook: Volume 1 (In Two Volumes). CRC Press, 1992. pp. 447–448. ISBN 0824786696.
- "Define and use K values, relative volatility, and x-y diagrams". The Pillars Curriculum for Chemical Engineering.
- McKetta Jr., John J. (24 September 1992). Unit Operations Handbook: Volume 1 (In Two Volumes). CRC Press, 1992. pp. 447–448. ISBN 0824786696.
- Thammasat University. "AE 335 Charts AE + SI" (PDF).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.