Decamethylferrocene
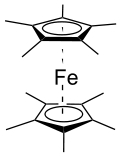
Decamethylferrocene or bis(pentamethylcyclopentadienyl)iron(II) is a chemical compound with formula Fe(C
5(CH
3)
5)
2 or C
20H
30Fe. It is a sandwich compound, whose molecule has an iron(II) cation Fe2+ attached by coordination bonds between two pentamethylcyclopentadienyl anions (Cp*−, (CH
3)
5C−
5). It can also be viewed as a derivative of ferrocene, with a methyl group replacing each hydrogen atom of its cyclopentadienyl rings. The name and formula are often abbreviated to DmFc,[3] Me
10Fc [4] or FeCp*
2.[5]
 | |
| Names | |
|---|---|
| IUPAC name
Bis(η5-pentamethylcyclopentadienyl)iron(II) | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.116.086 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H30Fe | |
| Molar mass | 326.305 g·mol−1 |
| Appearance | Yellow crystalline solid[1] |
| Melting point | 291 to 295 °C (556 to 563 °F; 564 to 568 K)[1] |
| 413 K, 5.3 Pa[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This compound is a yellow crystalline solid that is used in chemical laboratories as a weak reductant. The iron(II) core is easily oxidized to iron(III), yielding the monovalent cation decamethylferrocenium, and even to higher oxidation states.
Preparation
Decamethylferrocene is prepared in the same manner as ferrocene from pentamethylcyclopentadiene. This method can be used to produce other decamethylcyclopentadienyl sandwich compounds.[1]
- 2 Li(C5Me5) + FeCl2 → Fe(C5Me5)2 + 2 LiCl
The product can be purified by sublimation. FeCp*
2 has staggered Cp* rings. The average distance between iron and each carbon is approximately 2.050 Å. This structure has been confirmed by X-ray crystallography.[6]
Redox reactions
Like ferrocene, decamethylferrocene forms a stable cation because Fe(II) is easily oxidized to Fe(III). Because of the electron donating methyl groups on the Cp* groups, decamethylferrocene is more reducing than is ferrocene. In a solution of acetonitrile the reduction potential for the [FeCp*
2]+/0
couple is −0.48 V compared to a [FeCp
2]0/+
reference (−0.59 V vs Fc/Fc+ in CH
2Cl
2).[2] Oxygen is reduced to hydrogen peroxide by decamethylferrocene in acidic solution.[7]
Using powerful oxidants (e.g. SbF
5 or AsF
5 in SO
2, or XeF+/Sb
2F−
11 in HF/SbF
5) decamethylferrocene is oxidized to a stable dication with an iron(IV) core. In the Sb
2F−
11 salt, the Cp* rings are parallel. In contrast, a tilt angle of 17° between the Cp* rings is observed in the crystal structure of the SbF−
6 salt.[5]
References
- King, R. B.; Bisnette, M. B. (1967). "Organometallic Chemistry of the Transition Metals XXI. Some π-pentamethylcyclopentadienyl Derivatives of Various Transition Metals". J. Organomet. Chem. 8 (2): 287–297. doi:10.1016/S0022-328X(00)91042-8.
- Connelly, N.; Geiger, W. E. (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- Torriero, Angel A.J. (2014). "Characterization of decamethylferrocene and ferrocene in ionic liquids: argon and vacuum effect on their electrochemical properties". Electrochimica Acta. 137: 235–244. doi:10.1016/j.electacta.2014.06.005.
- Noviandri, Indra; Brown, Kylie N.; Fleming, Douglas S.; Gulyas, Peter T.; Lay, Peter A.; Masters, Anthony F.; Phillips, Leonidas (1 August 1999). "The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer". The Journal of Physical Chemistry B. 103 (32): 6713–6722. doi:10.1021/jp991381+. ISSN 1520-6106.
- Malischewski, M.; Adelhardt, M.; Sutter, J.; Meyer, K.; Seppelt, K. (2016). "Isolation and structural and electronic characterization of salts of the decamethylferrocene dication". Science. 353 (6300): 678–682. Bibcode:2016Sci...353..678M. doi:10.1126/science.aaf6362. PMID 27516596.
- Freyberg, Derek P.; Robbins, John L.; Raymond, Kenneth N.; Smart, James C. (1979). "Crystal and molecular structures of decamethylmanganocene and decamethylferrocene. Static Jahn-Teller distortion in a metallocene". J. Am. Chem. Soc. 101 (4): 892–897. doi:10.1021/ja00498a017.
- Su, Bin; Hatay, Imren; Ge, Pei Yu; Mendez, Manuel; Corminboeuf, Clemence; Samec, Zdenek; Ersoz, Mustafa; Girault, Hubert H. (2010). "Oxygen and proton reduction by decamethylferrocene in non-aqueous acidic media". Chem. Commun. 46 (17): 2918–2919. doi:10.1039/B926963K. PMID 20386822.