Degeneracy (biology)
Within biological systems, degeneracy occurs when structurally dissimilar components/modules/pathways can perform similar functions (i.e. are effectively interchangeable) under certain conditions, but perform distinct functions in other conditions.[1][2] Degeneracy is thus a relational property that requires comparing the behavior of two or more components. In particular, if degeneracy is present in a pair of components, then there will exist conditions where the pair will appear functionally redundant but other conditions where they will appear functionally distinct.[1][3]
Note that this use of the term has practically no relevance to the questionably meaningful concept of evolutionarily degenerate populations that have lost ancestral functions.
Biological examples
Examples of degeneracy are found in the genetic code, when many different nucleotide sequences encode the same polypeptide; in protein folding, when different polypeptides fold to be structurally and functionally equivalent; in protein functions, when overlapping binding functions and similar catalytic specificities are observed; in metabolism, when multiple, parallel biosynthetic and catabolic pathways may coexist. More generally, degeneracy is observed in proteins of every functional class (e.g. enzymatic, structural, or regulatory),[4][5] protein complex assemblies,[6] ontogenesis,[7] the nervous system,[8] cell signalling (crosstalk) and numerous other biological contexts reviewed in.[1]
Contribution to robustness
Degeneracy contributes to the robustness of biological traits through several mechanisms. Degenerate components compensate for one another under conditions where they are functionally redundant, thus providing robustness against component or pathway failure. Because degenerate components are somewhat different, they tend to harbor unique sensitivities so that a targeted attack such as a specific inhibitor is less likely to present a risk to all components at once.[3] There are numerous biological examples where degeneracy contributes to robustness in this way. For instance, gene families can encode for diverse proteins with many distinctive roles yet sometimes these proteins can compensate for each other during lost or suppressed gene expression, as seen in the developmental roles of the adhesins gene family in Saccharomyces.[9] Nutrients can be metabolized by distinct metabolic pathways that are effectively interchangeable for certain metabolites even though the total effects of each pathway are not identical.[10][11] In cancer, therapies targeting the EGF receptor are thwarted by the co-activation of alternate receptor tyrosine kinases (RTK) that have partial functional overlap with the EGF receptor (and are therefore degenerate), but are not targeted by the same specific EGF receptor inhibitor.[12][13] Other examples from various levels of biological organization can be found in.[1]
Theory
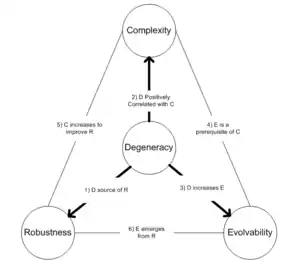
Several theoretical developments have outlined links between degeneracy and important biological measurements related to robustness, complexity, and evolvability. These include:
- Theoretical arguments supported by simulations have proposed that degeneracy can lead to distributed forms of robustness in protein interaction networks.[14] Those authors suggest that similar phenomena is likely to arise in other biological networks and potentially may contribute to the resilience of ecosystems as well.
- Tononi et al. have found evidence that degeneracy is inseparable from the existence of hierarchical complexity in neural populations.[8] They argue that the link between degeneracy and complexity is likely to be much more general.
- Fairly abstract simulations have supported the hypothesis that degeneracy fundamentally alters the propensity for a genetic system to access novel heritable phenotypes[15] and that degeneracy could therefore be a precondition for open-ended evolution.
- The three hypotheses above have been integrated in[3] where they propose that degeneracy plays a central role in the open-ended evolution of biological complexity. In the same article, it was argued that the absence of degeneracy within many designed (abiotic) complex systems may help to explain why robustness appears to be in conflict with flexibility and adaptability, as seen in software, systems engineering, and artificial life.[3]
See also
References
- Edelman and Gally; Gally, J. A. (2001). "Degeneracy and complexity in biological systems". Proceedings of the National Academy of Sciences, USA. 98 (24): 13763–13768. Bibcode:2001PNAS...9813763E. doi:10.1073/pnas.231499798. PMC 61115. PMID 11698650.
- Mason, Paul H. (2 January 2015). "Degeneracy: Demystifying and destigmatizing a core concept in systems biology". Complexity. 20 (3): 12–21. Bibcode:2015Cmplx..20c..12M. doi:10.1002/cplx.21534.
- Whitacre (2010). "Degeneracy: a link between evolvability, robustness and complexity in biological systems". Theoretical Biology and Medical Modelling. 7 (6): 6. arXiv:0910.2586. Bibcode:2009arXiv0910.2586W. doi:10.1186/1742-4682-7-6. PMC 2830971. PMID 20167097.
- Atamas (2005). "Les affinités électives". Pour la Science. 46: 39–43.
- Wagner (2000). "The role of population size, pleiotropy and fitness effects of mutations in the evolution of overlapping gene functions". Genetics. 154 (3): 1389–1401. PMC 1461000. PMID 10757778.
- Kurakin (2009). "Scale-free flow of life: on the biology, economics, and physics of the cell". Theoretical Biology and Medical Modelling. 6 (1): 6. doi:10.1186/1742-4682-6-6. PMC 2683819. PMID 19416527.
- Newman (1994). "Generic physical mechanisms of tissue morphogenesis: A common basis for development and evolution". Journal of Evolutionary Biology. 7 (4): 480. doi:10.1046/j.1420-9101.1994.7040467.x.
- Tononi; Sporns, O.; Edelman, G. M.; et al. (1999). "Measures of degeneracy and redundancy in biological networks". Proceedings of the National Academy of Sciences, USA. 96 (6): 3257–3262. Bibcode:1999PNAS...96.3257T. doi:10.1073/pnas.96.6.3257. PMC 15929. PMID 10077671.
- Guo; Styles, C. A.; Feng, Q.; Fink, G. R.; et al. (2000). "A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating". Proceedings of the National Academy of Sciences, USA. 97 (22): 12158–12163. Bibcode:2000PNAS...9712158G. doi:10.1073/pnas.220420397. PMC 17311. PMID 11027318.
- Kitano (2004). "Biological robustness". Nature Reviews Genetics. 5 (11): 826–837. doi:10.1038/nrg1471. PMID 15520792.
- Ma and Zeng; Zeng, AP (2003). "The connectivity structure, giant strong component and centrality of metabolic networks". Bioinformatics. 19 (11): 1423–1430. doi:10.1093/bioinformatics/btg177. PMID 12874056.
- Huang; Mukasa, A.; Bonavia, R.; Flynn, R. A.; Brewer, Z. E.; Cavenee, W. K.; Furnari, F. B.; White, F. M.; et al. (2007). "Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma". Proceedings of the National Academy of Sciences. 104 (31): 12867–72. Bibcode:2007PNAS..10412867H. doi:10.1073/pnas.0705158104. PMC 1937558. PMID 17646646.
- Stommel; Kimmelman, AC; Ying, H; Nabioullin, R; Ponugoti, AH; Wiedemeyer, R; Stegh, AH; Bradner, JE; et al. (2007). "Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies". Science. 318 (5848): 287–90. Bibcode:2007Sci...318..287S. doi:10.1126/science.1142946. PMID 17872411.
- Whitacre and Bender; Bender, Axel (2010). "Networked buffering: a basic mechanism for distributed robustness in complex adaptive systems". Theoretical Biology and Medical Modelling. 7 (20): 20. doi:10.1186/1742-4682-7-20. PMC 2901314. PMID 20550663.
- Whitacre and Bender; Bender, A (2010). "Degeneracy: a design principle for achieving robustness and evolvability". Journal of Theoretical Biology. 263 (1): 143–153. arXiv:0907.0510. doi:10.1016/j.jtbi.2009.11.008. PMID 19925810.
Further reading
Because there are many distinct types of systems that undergo heritable variation and selection (see Universal Darwinism), degeneracy has become a highly interdisciplinary topic. The following provides a brief roadmap to the application and study of degeneracy within different disciplines.
- Hebets E. A., Barron A. B., Balakrishnan C. N., Hauber M. E., Mason P. H., Hoke K. L. (2016). "A systems approach to animal communication". Proc. R. Soc. B. 283 (1826): 20152889. doi:10.1098/rspb.2015.2889. PMC 4810859. PMID 26936240.CS1 maint: multiple names: authors list (link)
- Downey G (2012). "Cultural variation in rugby skills: A preliminary neuroanthropological report". Annals of Anthropological Practice. 36 (1): 26–44. doi:10.1111/j.2153-9588.2012.01091.x.
- Atamas S., Bell J. (2009). "Degeneracy-Driven Self-Structuring Dynamics in Selective Repertoires". Bulletin of Mathematical Biology. 71 (6): 1349–1365. doi:10.1007/s11538-009-9404-z. PMC 3707519. PMID 19337776.
- Maleszka R., Mason P.H., Barron A.B. (2014). "Epigenomics and the concept of degeneracy in biological systems". Briefings in Functional Genomics. 13 (3): 191–202. doi:10.1093/bfgp/elt050.CS1 maint: multiple names: authors list (link)
History and philosophy of science
- Mason P.H. (2010). "Degeneracy at Multiple Levels of Complexity". Biological Theory. 5 (3): 277–288. doi:10.1162/biot_a_00041.
- Solé R.V., Ferrer-Cancho R., Montoya J.M., Valverde S. (2002). "Selection, tinkering, and emergence in complex networks" (PDF). Complexity. 8 (1): 20–33. Bibcode:2002Cmplx...8a..20S. doi:10.1002/cplx.10055.CS1 maint: multiple names: authors list (link)
- Whitacre J.M., Bender A. (2010). "Networked buffering: a basic mechanism for distributed robustness in complex adaptive systems". Theoretical Biology and Medical Modelling. 7 (20). arXiv:0912.1961. Bibcode:2009arXiv0912.1961W. doi:10.1186/1742-4682-7-20. PMC 2901314. PMID 20550663.
- Mason P.H. (2015). "Degeneracy: Demystifying and destigmatizing a core concept in systems biology". Complexity. 20 (3): 12–21. Bibcode:2015Cmplx..20c..12M. doi:10.1002/cplx.21534.
- Mason P.H., Domínguez D. J.F., Winter B., Grignolio A. (2015). "Hidden in plain view: degeneracy in complex systems". BioSystems. 128: 1–8. doi:10.1016/j.biosystems.2014.12.003.CS1 maint: multiple names: authors list (link)
- Edelman G.M., Gally J.A. (2001). "Degeneracy and complexity in biological systems". Proceedings of the National Academy of Sciences, USA. 98 (24): 13763–13768. Bibcode:2001PNAS...9813763E. doi:10.1073/pnas.231499798. PMC 61115. PMID 11698650.
- Whitacre J.M. (2010). "Degeneracy: a link between evolvability, robustness and complexity in biological systems". Theoretical Biology and Medical Modelling. 7 (6). arXiv:0910.2586. Bibcode:2009arXiv0910.2586W. doi:10.1186/1742-4682-7-6. PMC 2830971. PMID 20167097.
- Whitacre J.M., Bender A. (2010). "Degeneracy: a design principle for achieving robustness and evolvability". Journal of Theoretical Biology. 263 (1): 143–53. arXiv:0907.0510. doi:10.1016/j.jtbi.2009.11.008. PMID 19925810.
- Whitacre J.M., Atamas S.P. (2011). "The Diversity Paradox: How Nature Resolves an Evolutionary Dilemma". arXiv:1112.3115. Bibcode:2011arXiv1112.3115W. Cite journal requires
|journal=(help)
- Cohn M (2005). "Degeneracy, mimicry and crossreactivity in immune recognition". Molecular Immunology. 42 (5): 651–655. doi:10.1016/j.molimm.2004.09.010.
- Cohen, I.R., U. Hershberg, and S. Solomon, 2004 Antigen-receptor degeneracy and immunological paradigms. Molecular Immunology, . 40(14–15) pp. 993–996.
- Tieri, P., G.C. Castellani, D. Remondini, S. Valensin, J. Loroni, S. Salvioli, and C. Franceschi, Capturing degeneracy of the immune system. In Silico Immunology. Springer, 2007.
- Tieri P., Grignolio A., Zaikin A., Mishto M., Remondini D., Castellani G.C., Franceschi C. (2010). "Network, degeneracy and bow tie. Integrating paradigms and architectures to grasp the complexity of the immune system". Theor Biol Med Model. 7: 32. doi:10.1186/1742-4682-7-32. PMC 2927512. PMID 20701759.CS1 maint: multiple names: authors list (link)
Artificial life, Computational intelligence
- Andrews, P.S. and J. Timmis, A Computational Model of Degeneracy in a Lymph Node. Lecture Notes in Computer Science, 2006. 4163: p. 164.
- Mendao, M., J. Timmis, P.S. Andrews, and M. Davies. The Immune System in Pieces: Computational Lessons from Degeneracy in the Immune System. in Foundations of Computational Intelligence (FOCI). 2007.
- Whitacre, J.M. and A. Bender. Degenerate neutrality creates evolvable fitness landscapes. in WorldComp-2009. 2009. Las Vegas, Nevada, USA.
- Whitacre, J.M., P. Rohlfshagen, X. Yao, and A. Bender. The role of degenerate robustness in the evolvability of multi-agent systems in dynamic environments. in PPSN XI. 2010. Kraków, Poland.
- Macia J., Solé R. (2009). "Distributed robustness in cellular networks: insights from synthetic evolved circuits". Journal of the Royal Society Interface. 6 (33): 393–400. doi:10.1098/rsif.2008.0236. PMC 2658657. PMID 18796402.
- Fernandez-Leon, J.A. (2011). Evolving cognitive-behavioural dependencies in situated agents for behavioural robustness. BioSystems 106, pp. 94–110.[1]
- Fernandez-Leon, J.A. (2011). Behavioural robustness: a link between distributed mechanisms and coupled transient dynamics. BioSystems 105, Elsevier, pp. 49–61.[2]
- Fernandez-Leon, J.A. (2010). Evolving experience-dependent robust behaviour in embodied agents. BioSystems 103:1, Elsevier, pp. 45–56.[3]
- Price, C. and K. Friston, Degeneracy and cognitive anatomy. Trends in Cognitive Sciences, 2002. 6(10) pp. 416–421.
- Tononi, G., O. Sporns, and G.M. Edelman, Measures of degeneracy and redundancy in biological networks. Proceedings of the National Academy of Sciences, USA, 1999. 96(6) pp. 3257–3262.
- Mason, P.H. (2014) What is normal? A historical survey and neuroanthropological perspective, in Jens Clausen and Neil Levy. (Eds.) Handbook of Neuroethics, Springer, pp. 343–363.
- Winter B (2014). "Spoken language achieves robustness and evolvability by exploiting degeneracy and neutrality". BioEssays. 36 (10): 960–967. doi:10.1002/bies.201400028. PMID 25088374.
- Tian, T., S. Olson, J.M. Whitacre, and A. Harding, The origins of cancer robustness and evolvability. Integrative Biology, 2011. 3: pp. 17–30.
- Lehky, S., Peer Evaluation and Selection Systems: Adaptation and Maladaptation of Individuals and Groups through Peer Review. 2011: BioBitField Press.
External links
- Fernandez-Leon, J.A. (2011). "Evolving cognitive-behavioural dependencies in situated agents for behavioural robustness". BioSystems. 106 (2–3): 94–110. doi:10.1016/j.biosystems.2011.07.003. PMID 21840371.
- Fernandez-Leon, J.A. (2011). "Behavioural robustness: a link between distributed mechanisms and coupled transient dynamics". BioSystems. 105 (1): 49–61. doi:10.1016/j.biosystems.2011.03.006. PMID 21466836.
- Fernandez-Leon, J.A. (2010). "Evolving experience-dependent robust behaviour in embodied agents". BioSystems. 103 (1): 45–56. doi:10.1016/j.biosystems.2010.09.010. PMID 20932875.