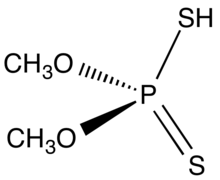
Dimethyl dithiophosphoric acid
Dimethyl dithiophosphoric acid is the organophosphorus compound with the formula (CH3O)2PS2H. It is the processor for production of the organothiophosphate insecticide Malathion. Although samples can appear dark, the compound is a colorless, distillable liquid.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Dimethoxy-sulfanyl-sulfanylidene-λ5-phosphane | |
| Other names
O,O-Dimethyl dithiophosphoric acid; Dimethyl dithiophosphate; Dimethyl phosphorodithioate; Dimethyl ester of phosphorodithioic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.010.958 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H7O2PS2 | |
| Molar mass | 158.17 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 62–64 °C (144–147 °F; 335–337 K) 0.5 mm Hg |
| Hazards | |
EU classification (DSD) (outdated) |
Flammable (F) Toxic (T) Corrosive (C) |
| R-phrases (outdated) | R23/24/25 R34 R41 |
| S-phrases (outdated) | S23 S26 S36/37/39 S45 |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is prepared by treating phosphorus pentasulfide with methanol:[2]
- P2S5 + 4 CH3OH → 2 (CH3O)2PS2H + H2S
References
- J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a19_545.pub2
- Lefferts, J. L.; Molloy, K. C.; Zuckerman, J. J.; Haiduc, I.; Guta, C.; Ruse, D., "Oxy and thio phosphorus acid derivatives of tin. 1. Triorganotin(IV) dithiophosphate esters", Inorganic Chemistry 1980, volume 19, 1662-1670. doi:10.1021/ic50208a046
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
