Distamycin
Distamycin is a polyamide-antibiotic, which acts as a minor groove binder, binding to the small furrow of the double helix.[1]
 | |
| Names | |
|---|---|
| IUPAC name
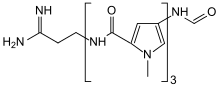
N-{5-[(5-{[(3Z)-3-Amino-3-iminopropyl]carbamoyl}-1-methyl-1H-pyrrol-3-yl)carbamoyl]-1-methyl-1H-pyrrol-3-yl}-4-formamido-1-methyl-1H-pyrrole-2-carboxamide | |
| Other names
Distamycin A, Herperetin, Stallimycin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.026.823 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C22H27N9O4 | |
| Molar mass | 481.508 g/mol |
| Appearance | White powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
Distamycin is a pyrrole-amidine antibiotic and analogous to netropsin and the class of lexitropsins. As opposed to netropsin, distamycin contains three N-methyl-pyrrole units. It is harvested from Streptomyces netropsis that also produces netropsin. Distamycin prefers AT-rich DNA-sequences and tetrades of [TGGGGT]4.[2][3]
Distamycin inhibits the transcription and increases the activity of the topoisomerase II.[4][5] Derivates from distamycin are used as alkylating antineoplastic agents to combat tumours.[1][6] Derivates with fluorophores are used as fluorescent tags for double-stranded DNA.[7]
The compound is hygroscopic, and sensible to light, freeze and hydrolysis. Its molar attenuation coefficient is 37,000 M−1 cm−1 at a wavelength of 303 nm.
See also
References
- Barrett, Michael P.; Gemmell, Curtis G.; Suckling, Colin J. (2013). "Minor groove binders as anti-infective agents". Pharmacology & Therapeutics. 139 (1): 12–23. doi:10.1016/j.pharmthera.2013.03.002. PMID 23507040.
- Kopka, M. L.; Yoon, C.; Goodsell, D.; Pjura, P.; Dickerson, R. E. (1985). "The molecular origin of DNA-drug specificity in netropsin and distamycin". Proceedings of the National Academy of Sciences of the United States of America. 82 (5): 1376–80. Bibcode:1985PNAS...82.1376K. doi:10.1073/pnas.82.5.1376. PMC 397264. PMID 2983343.
- Pagano, Bruno; Fotticchia, Iolanda; De Tito, Stefano; Mattia, Carlo A.; Mayol, Luciano; Novellino, Ettore; Randazzo, Antonio; Giancola, Concetta (2010). "Selective Binding of Distamycin a Derivative to G-Quadruplex Structure [d(TGGGGT)]4". Journal of Nucleic Acids. 2010: 1–7. doi:10.4061/2010/247137. PMC 2915651. PMID 20725616.
- Majumder, Parijat; Banerjee, Amrita; Shandilya, Jayasha; Senapati, Parijat; Chatterjee, Snehajyoti; Kundu, Tapas K.; Dasgupta, Dipak (2013). "Minor Groove Binder Distamycin Remodels Chromatin but Inhibits Transcription". PLOS ONE. 8 (2): e57693. Bibcode:2013PLoSO...857693M. doi:10.1371/journal.pone.0057693. PMC 3584068. PMID 23460895.
- Fesen, M.; Pommier, Y. (1989). "Mammalian topoisomerase II activity is modulated by the DNA minor groove binder distamycin in simian virus 40 DNA". The Journal of Biological Chemistry. 264 (19): 11354–9. PMID 2544590.
- Baraldi, Pier Giovanni; Preti, Delia; Fruttarolo, Francesca; Tabrizi, Mojgan Aghazadeh; Romagnoli, Romeo (2007). "Hybrid molecules between distamycin a and active moieties of antitumor agents". Bioorganic & Medicinal Chemistry. 15 (1): 17–35. doi:10.1016/j.bmc.2006.07.004. PMID 17081759.
- Vaijayanthi, Thangavel; Bando, Toshikazu; Pandian, Ganesh N.; Sugiyama, Hiroshi (2012). "Progress and Prospects of Pyrrole-Imidazole Polyamide-Fluorophore Conjugates as Sequence-Selective DNA Probes". ChemBioChem. 13 (15): 2170–2185. doi:10.1002/cbic.201200451. PMID 23023993. S2CID 1491900.