Dithietane
Dithietanes are saturated heterocyclic compounds that contain two divalent sulfur atoms and two sp3-hybridized carbon centers.[1][2] Two isomers are possible for this class of organosulfur compounds:
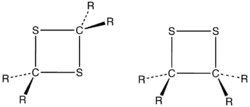
1,2-Dithietanes
1,2-dithietanes, 4-membered rings where the two sulfur atoms are adjacent, are very rare. The first stable 1,2-dithietane to be reported was the so-called dithiatopazine formed by intramolecular photodimerization of a dithiocarbonyl compound.[3] 1,2-Dithietanes are to be distinguished from 1,2-dithietes, containing two adjacent sulfur atoms and two sp2-hybridized carbon centers.
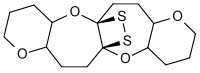
A stable 1,2-dithietane derivative is trans-3,4-diethyl-1,2-dithietane 1,1-dioxide, formed by the spontaneous dimerization of the lachrymatory agent syn-propanethial-S-oxide, found in onion.[4]
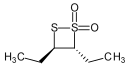
1,3-Dithietanes
In 1,3-dithietanes, the sulfur atoms are non-adjacent.[5] 1,3-Dithietane itself, a colorless, easily sublimed, crystalline, unpleasant-smelling solid with melting point 105-106 °C, was first prepared in 1976 by reaction of bis(chloromethyl) sulfoxide with sodium sulfide followed by THF-borane reduction of the first formed 1,3-dithietane 1-oxide, as shown in the scheme below.[6][7] Carbon-substituted 1,3-dithietanes are well known, with the first examples being described as early as 1872. Examples include 2,2,4,4-tetrachloro-1,3-dithietane, the photochemically-formed dimer of thiophosgene, and tetrakis(trifluoromethyl)-1,3-dithietane, [(CF3)2CS]2.[8]

Oxidized forms of 1,3-dithietane are well known, although they are often not prepared from the dithietane. Examples include the so-called zwiebelanes (2,3-dimethyl-5,6-dithiabicyclo[2.1.1]hexane S-oxides) from onion volatiles[9] and 1,3-dithietane 1,1,3,3-tetraoxide, the so-called sulfene dimer.[10]
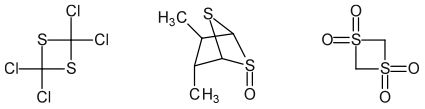
References
- Drabowicz, J; Lewkowski, J; Kudelska, W; Zając, A (2008). "Four-membered Rings with Two Sulfur Atoms". Comprehensive Heterocyclic Chemistry III. 2.18: 811–852. doi:10.1016/B978-008044992-0.00218-2.
- Zoller, U (1996). "Four-membered Rings with Two Sulfur Atoms". Comprehensive Heterocyclic Chemistry II. 1.35: 1113–1138. doi:10.1016/B978-008096518-5.00035-6.
- Nicolaou, KC; Hwang, CK; Duggan, ME; Carroll, PJ (1987). "Dithiatopazine. The first stable 1,2-dithietane". J. Am. Chem. Soc. 109 (12): 3801–3802. doi:10.1021/ja00246a059.
- Block, E; Bazzi, AA; Revelle, LK (1980). "The chemistry of sulfines. 6. Dimer of the onion lachrymatory factor: the first stable 1,2-dithietane derivative". J. Am. Chem. Soc. 102 (7): 2490–2491. doi:10.1021/ja00527a074.
- Luh, TY; Leung, MK (2007). "Product Subclass 2: 1,3-Dithietanes". Sci. Synth. 30: 203–219.
- Block, E; Corey, ER; Penn, RE; Renken, TL; Sherwin, PF (1976). "1,3-Dithietane". J. Am. Chem. Soc. 98 (18): 5715–5717. doi:10.1021/ja00434a061.
- Block, E; Corey, ER; Penn, RE; Renken, TL; Sherwin, PF; Bock, H; Hirabayashi, T; Mohmand, S; Solouki, B (1982). "Synthesis and Thermal Decomposition of 1,3-Dithietane and its S-Oxides". J. Am. Chem. Soc. 104 (11): 3119–3130. doi:10.1021/ja00375a030.
- Van Der Puy, M.; Anello, L. G. (1990). "Hexafluoroacetone". Organic Syntheses.; Collective Volume, 7, p. 251.
- Block, E; Thiruvazhi, M; Toscano, PJ; Bayer, T; Grisoni, S; Zhao, SH (1996). "Allium Chemistry: Structure, Synthesis, Natural Occurrence in Onion (Allium cepa), and Reactions of 2,3-Dimethyl-5,6-dithiabicyclo[2.1.1]hexane S-Oxides". J. Am. Chem. Soc. 118 (12): 2790–2798. doi:10.1021/ja951134t.
- Opitz, G; Mohl, HR (1969). "Disulfene". Angew. Chem. Int. Ed. 8 (1): 73. doi:10.1002/anie.196900731.