Dithiobenzoic acid
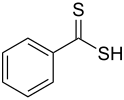
Dithiobenzoic acid is the organosulfur compound with the formula C6H5CS2H. It is a dithiocarboxylic acid, an analogue of benzoic acid, but more acidic and deeply colored.
 | |
| Names | |
|---|---|
| IUPAC name
Benzenecarbodithioic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.084 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H6S2 | |
| Molar mass | 154.25 g·mol−1 |
| Appearance | yellow-brown solid |
| Acidity (pKa) | 1.92[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
It can be prepared by sulfiding benzal chloride:[2]
- C6H5CCl3 + 4 KSH → C6H5CS2K + 3 KCl + 2 H2S
- C6H5CS2K + H+ → C6H5CS2H + K+
It also arises by the reaction of the Grignard reagent phenylmagnesium bromide with carbon disulfide, followed by acidification:[3]
- C6H5MgBr + CS2 → C6H5CS2MgBr
- C6H5CS2MgBr + HCl → C6H5CS2H + MgBrCl
It is about 100x more acidic than benzoic acid. Its conjugate base, dithiobenzoate, undergoes S-alkylation to give dithiocarboxylate esters.[2] Similarly, dithiobenzoate reacts with "soft" metal salts to give complexes, e.g. Fe(S2CC6H5)3 and Ni(S2CC6H5)2.
Chlorination of dithiobenzoic acid gives the thioacyl chloride C6H5C(S)Cl.

Structure of the trimer [Ni(S2CPh)2]3.[4]
References
- M. R. Crampton (1974). "Acidity and hydrogen-bonding". In Saul Patai (ed.). The Chemistry of the Thiol Group. Chichester: John Wiley & Sons Ltd. p. 402.
- Frederick Kurzer, Alexander Lawson (1962). "Thiobenzoylthioglycolic Acid". Org. Synth. 42: 100. doi:10.15227/orgsyn.042.0100.CS1 maint: uses authors parameter (link)
- J. Houben (1906). "Ueber Carbithiosäuren. I. Arylcarbithiosäuren" [About Carbothioic Acids I. Arylcarbothioic Acids]. Berichte der Deutschen Chemischen Gesellschaft. 39 (3): 3219–3233. doi:10.1002/cber.190603903140.
- Bonamico, M.; Dessy, G.; Fares, V.; Scaramuzza, L. (1975). "Structural Studies of Metal Complexes with Sulphur-Containing Bidentate Ligands. Part I. Crystal and Molecular Structures of Trimeric Bis-(dithiobenzoato)-nickel(II) and -palladium(II)". Journal of the Chemical Society, Dalton Transactions (21): 2250–2255. doi:10.1039/DT9750002250.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.