Dithiocarbamate
A dithiocarbamate is a functional group in organic chemistry. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only 1 oxygen is replaced the result is thiocarbamate).
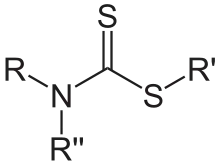
A common example is sodium diethyldithiocarbamate. Dithiocarbamates and their derivatives are widely used in the vulcanization of rubber.[1]
Formation
Many primary and secondary amines react with carbon disulfide and sodium hydroxide to form dithiocarbamate salts:[2]
- R2NH + CS2 + NaOH → R2NCS2−Na+ + H2O
Ammonia reacts with CS2 similarly:
- 2 NH3 + CS2 → H2NCS2−NH4+
Dithiocarbamate salts are pale colored solids that are soluble in water and polar organic solvents.
Reactions
Dithiocarbamates are readily S-alkylated. Thus, methyl dimethyldithiocarbamate can be prepared by methylation of the dithiocarbamate:[3]
- (CH3)2NCS2Na + (CH3O)2SO2 → (CH3)2NC(S)SCH3 + Na[CH3OSO3]
Oxidation of dithiocarbamates gives the thiuram disulfide:
- 2 R2NCS2− → [R2NC(S)S]2 + 2e−
Thiuram disulfides react with Grignard reagents to give esters of dithiocarbamic acid:[4]
- [R2NC(S)S]2 + R'MgX → R2NC(S)SR' + R2NCS2MgX
Dithiocarbamates react with transition metal salts to give a wide variety of transition metal dithiocarbamate complexes.
Structure and bonding
Dithiocarbamates is described by invoking resonance structures that emphasize the pi-donor properties of the amine group. This bonding arrangement is indicated by a short C–N distance and the coplanarity of the NCS2 core as well as the atoms attached to N.[5]
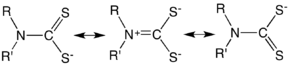
Because of the pi-donation from nitrogen, dithiocarbamates are more basic than structurally related anions such as dithiocarboxylates and xanthates. Consequently, they tend to bind as bidentate ligands. Another consequence of the C–N multiple bonding is that rotation about that bond is subject to a high barrier.
Applications
Zinc dithiocarbamates are used to modify the crosslinking of certain polyolefins with sulfur, a process called vulcanization. They are used as ligands for chelating metals.[6]
2Improved.png.webp)
Dithiocarbamates specifically ethylene bisdithiocarbamates (EBDCs), in the form of complexes with manganese (maneb), zinc (zineb) or a combination of manganese and zinc (mancozeb), have been used extensively as fungicides in agriculture from the 1940s.[7]
Dithiocarbamates also serve as anchoring groups for molecular species in the functionalization of semiconductor and metal surfaces.[9][10] In cadmium chalcogenide nanocrystals functionalized with dithiocarbamate ligands, the energetic alignment of the semiconductor's valence band and the HOMO level of the ligands has been shown to facilitate delocalization of holes from the nanocrystal core onto the ligand.[11][12] The hole-delocalizing properties of dithiocarbamates have been used to create ligands that stabilize the nanocrystals in solution while also mediating charge transfer through the ligand shell (which typically hinders this process).[13]
See also
References
- Engels, Hans-Wilhelm; et al. "Rubber, 4. Chemicals and Additives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_365.pub2.
- Rüdiger Schubart (2000). "Dithiocarbamic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_001. ISBN 3527306730.
- A. D. Ainley, W. H. Davies, H. Gudgeon, J. C. Harland and W. A. Sexton (1944). "The Constitution of the So-Called Carbothialdines and the Preparation of Some Homologous Compounds". J. Chem. Soc.: 147–152. doi:10.1039/JR9440000147.CS1 maint: uses authors parameter (link)
- John R. Grunwell (1970). "Reaction of Grignard Reagents with Tetramethylthiuram Disulfide [yielding dithiocarbamates]". J. Org. Chem. 35 (5): 1500–1501. doi:10.1021/jo00830a052.
- Coucouvanis, Dimitri (1979). "The chemistry of the dithioacid and 1,1-dithiolate complexes, 1968–1977". Prog. Inorg. Chem. Progress in Inorganic Chemistry. 26: 301–469. doi:10.1002/9780470166277.ch5. ISBN 9780470166277.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- "A Short History of Fungicides". The American Phytopathological Society. Archived from the original on 16 April 2016. Retrieved 10 May 2016.
- Theo Mang, Jürgen Braun, Wilfried Dresel, Jürgen Omeis (2011). "Lubricants, 2. Components". Ullmanns Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.o15_o04. ISBN 978-3527306732.CS1 maint: uses authors parameter (link)
- Zhao, Yan; Pérez-Segarra, Waleska; Shi, Qicun; Wei, Alexander (May 2005). "Dithiocarbamate Assembly on Gold". Journal of the American Chemical Society. 127 (20): 7328–7329. doi:10.1021/ja050432f. ISSN 0002-7863. PMC 1766936. PMID 15898778.
- Wang, Jun; Xu, Jun; Goodman, Matthew D.; Chen, Ying; Cai, Min; Shinar, Joseph; Lin, Zhiqun (2008). "A simple biphasic route to water soluble dithiocarbamate functionalized quantum dots". Journal of Materials Chemistry. 18 (27): 3270. doi:10.1039/b803618g. ISSN 0959-9428.
- Frederick, Matthew T.; Amin, Victor A.; Cass, Laura C.; Weiss, Emily A. (2011-12-14). "A Molecule to Detect and Perturb the Confinement of Charge Carriers in Quantum Dots". Nano Letters. 11 (12): 5455–5460. Bibcode:2011NanoL..11.5455F. doi:10.1021/nl203222m. ISSN 1530-6984.
- Lian, Shichen; Weinberg, David J.; Harris, Rachel D.; Kodaimati, Mohamad S.; Weiss, Emily A. (2016-06-28). "Subpicosecond Photoinduced Hole Transfer from a CdS Quantum Dot to a Molecular Acceptor Bound Through an Exciton-Delocalizing Ligand". ACS Nano. 10 (6): 6372–6382. doi:10.1021/acsnano.6b02814. ISSN 1936-0851. PMID 27281685.
- Lee, Jonathan R.; Li, Wei; Cowan, Alexander J.; Jäckel, Frank (2017-07-20). "Hydrophilic, Hole-Delocalizing Ligand Shell to Promote Charge Transfer from Colloidal CdSe Quantum Dots in Water". The Journal of Physical Chemistry C. 121 (28): 15160–15168. doi:10.1021/acs.jpcc.7b02949. ISSN 1932-7447.