E7070
E7070 is a novel chloroindolyl sulfonamide cell cycle that exhibits potent antitumoractivity in vitro (in a laboratory) and in vivo (in the body).[1][2][3] This compound affects cell cycle progression in human tumor cells and is most commonly used to treat cancers such as melanomas and tumor cells but is also used to treat blood-borne cancers such as leukemia.[4]
 | |
| Names | |
|---|---|
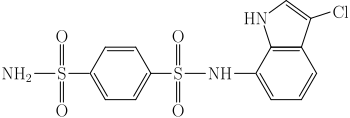
| IUPAC name
N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonamide | |
| Other names
Indisulam | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H12ClN3O4S2 | |
| Molar mass | 385.84 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Fukuoka, K., Usuda, J., Iwamoto, Y. et al. . (2001). "Mechanisms of Action of the Novel Sulfonamide Anticancer Agent E7070 on Cell Cycle Progression in Human Non-Small Cell Lung Cancer Cells". Invest New Drugs. 19 (3): 219–27. doi:10.1023/A:1010608317361. PMID 11561678. S2CID 26100991.CS1 maint: uses authors parameter (link)
- Y, Ozawa; Nh, Sugi; T, Nagasu; T, Owa; T, Watanabe; N, Koyanagi; H, Yoshino; K, Kitoh; K, Yoshimatsu (November 2001). "E7070, a Novel Sulphonamide Agent With Potent Antitumour Activity in Vitro and in Vivo". European Journal of Cancer (Oxford, England : 1990). 37 (17): 2275–82. doi:10.1016/s0959-8049(01)00275-1. PMID 11677118.
- E, Raymond; Ww, ten Bokkel Huinink; J, Taïeb; Jh, Beijnen; S, Faivre; J, Wanders; M, Ravic; P, Fumoleau; Jp, Armand (2002-08-15). "Phase I and Pharmacokinetic Study of E7070, a Novel Chloroindolyl Sulfonamide Cell-Cycle Inhibitor, Administered as a One-Hour Infusion Every Three Weeks in Patients With Advanced Cancer". Journal of Clinical Oncology. 20 (16): 3508–21. doi:10.1200/JCO.2002.09.030. PMID 12177112.
- Rita Assi, MD, Hagop M. Kantarjian, MD, Jorge E. Cortes, MD, Tapan Kadia, MD, Naveen Pemmaraju, MD, Elias J. Jabbour, MD, Nitin Jain, MD, Naval Daver, MD, Taisuke Uehara, Takashi Owa, PhD, Gautam Borthakur, MD (2001). "Final Results of Phase 2, Open-Label Study of E7070, Idarubicin and Cytarabine in Patients (Pts) with Relapsed or Refractory (R/R) Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndrome (MDS)". Blood. 130 (supplement 1).CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.