EDDHA
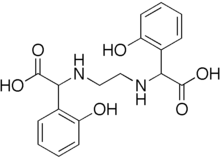
EDDHA or ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) is a chelating agent. Like EDTA, it binds metal ions as a hexadentate ligand, using two amines, two phenolate centers, and two carboxylates as the six binding sites. The complexes are typically anionic. The ligand itself is a white, water soluble powder. Both the free ligand and its tetraanionic chelating agent are abbreviated EDDHA. In contrast to EDDHA, most related aminopolycarboxylic acid chelating agents feature tertiary amines and few have phenolate groups.
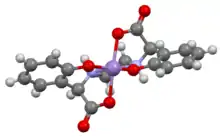 Structure of anion [Mn(EDDHA)]−, which is representative of related C2-symmetric complexes.[1]
Structure of anion [Mn(EDDHA)]−, which is representative of related C2-symmetric complexes.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2-[2-[[2-Hydroxy-1-(2-hydroxyphenyl)-2-oxoethyl]amino]ethylamino]-2-(2-hydroxyphenyl)acetic acid | |
| Other names
Ethylenediamine-N,N'-bis(2-hydroxyphenylacetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.013.296 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H20N2O6 | |
| Molar mass | 360.366 g·mol−1 |
| Appearance | White solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
It is produced by the multicomponent reaction of phenol, glyoxalic acid, and ethylenediamine. In this process, the initial Schiff base condensate alkylates the phenol.[2] Related ligands can be prepared more efficiently using para-cresol.[3]
Uses
It is used to mobilize metal ions analogously to the use of EDTA.[4][5]
EDDHA has been used in phytoextraction of lead from contaminated soils.[6] It degrades with release of salicylic acid.[7]
References
- Bihari, S.; Smith, P. A.; Parsons, S.; Sadler, P. J. (2002). "Stereoisomers of Mn(III) Complexes of Ethylenebis[(O-Hydroxyphenyl)Glycine]". Inorganica Chimica Acta. 331: 310–317. doi:10.1016/S0020-1693(02)00690-4.CS1 maint: uses authors parameter (link)
- Hernandez-Apaolaza, Lourdes; Garcia-Marco, Sonia; Nadal, Paloma; Lucena, Juan J.; Sierra, Miguel A.; Gomez-Gallego, Mar; Ramirez-Lopez, Pedro; Escudero, Rosa (2006). "Structure and Fertilizer Properties of Byproducts Formed in the Synthesis of EDDHA". Journal of Agricultural and Food Chemistry. 54 (12): 4355–4363. doi:10.1021/jf0605749.CS1 maint: uses authors parameter (link)
- Yunta, Felipe; Garcia-Marco, Sonia; Lucena, Juan J.; Gomez-Gallego, Mar; Alcazar, Roberto; Sierra, Miguel A. (2003). "Chelating Agents Related to Ethylenediamine Bis(2-hydroxyphenyl)acetic Acid (EDDHA): Synthesis, Characterization, and Equilibrium Studies of the Free Ligands and Their Mg2+, Ca2+, Cu2+, and Fe3+ Chelates". Inorganic Chemistry. 42: 5412–5421. doi:10.1021/ic034333j. PMID 12924915.CS1 maint: uses authors parameter (link)
- Diarra MS, Petitclerc D, Lacasse P (2002). "Response of Staphylococcus aureus isolates from bovine mastitis to exogenous iron sources". J. Dairy Sci. 85 (9): 2141–8. doi:10.3168/jds.S0022-0302(02)74292-6. PMID 12362445.
- Sritharan M, Asuthkar S (2004). "Iron-regulated proteins (IRPS) of leptospira biflexa serovar Patoc strain Patoc I". Indian Journal of Medical Microbiology. 22 (2): 92–6. PMID 17642703.
- Huang, Jianwei W.; Chen, Jianjun; Berti, William R.; Cunningham, Scott D. (1997). "Phytoremediation of Lead-Contaminated Soils: Role of Synthetic Chelates in Lead Phytoextraction". Environmental Science and Technology. 31 (3): 800–805. Bibcode:1997EnST...31..800H. doi:10.1021/ES9604828.CS1 maint: uses authors parameter (link)
- Pieterse, Arnold H. (2013). "Is flowering in Lemnaceae stress-induced? A review". Aquatic Botany. 104: 1–4. doi:10.1016/j.aquabot.2012.08.002.