Elaiomycin
Elaiomycin is an antimicrobial chemical compound, classified as an azoxyalkene, which was first isolated from Streptomyces in 1954.[1][2] A laboratory synthesis of elaiomycin was reported in 1977.[3]
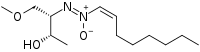 | |
| Names | |
|---|---|
| IUPAC name
(2S,3S)-(3-Hydroxy-1-methoxybutan-2-yl)imino-[(E)-oct-1-enyl]-oxidoazanium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| Properties | |
| C13H26N2O3 | |
| Molar mass | 258.362 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
A variety related compounds, collectively called elaiomycins, have also been reported.[4][5]
References
- Haskell, Theodore H.; Ryder, Albert; Bartz, Quentin R. (1954). "Elaiomycin, a new tuberculostatic antibiotic; isolation and chemical characterization". Antibiotics and Chemotherapy. 4 (2): 141–144. PMID 24542889.
- Erlich, J.; Anderson, LE; Coffey, GL; Feldman, WH; Fisher, MW; Hillegas, AB; Karlson, AG; Knudsen, MP; Weston, JK; Youmans, AS; Youmans, GP (1954). "Elaiomycin, a new tuberculostatic antibiotic; biologic studies". Antibiotics and Chemotherapy. 4 (3): 338–342. PMID 24542957.
- Moss RA, Matsuo M (March 1977). "The synthesis of elaiomycin, a naturally occurring azoxyalkene". Journal of the American Chemical Society. 99 (5): 1643–5. doi:10.1021/ja00447a060. PMID 839012.
- Helaly, Soleiman E.; Pesic, Alexander; Fiedler, Hans-Peter; Süssmuth, Roderich D. (2011). "Elaiomycins B and C: Alkylhydrazide Antibiotics from Streptomyces sp. BK 190". Organic Letters. 13 (5): 1052–1055. doi:10.1021/ol1031014. PMID 21309518.
- Ding L, Ndejouong Ble S, Maier A, Fiebig HH, Hertweck C (October 2012). "Elaiomycins D-F, antimicrobial and cytotoxic azoxides from Streptomyces sp. strain HKI0708". Journal of Natural Products. 75 (10): 1729–34. doi:10.1021/np300329m. PMID 23013356.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.