Electrodeionization
Electrodeionization (EDI) is a water treatment technology that utilizes electricity, ion exchange membranes and resin to deionize water and separate dissolved ions (impurities) from water. It differs from other water purification technologies in that it is done without the use of chemical treatments and is usually a polishing treatment to reverse osmosis (RO). There are also EDI units that are often referred to as continuous electrodeionization (CEDI) since the electric current regenerates the resin mass continuously. CEDI technique can achieve very high purity, with conductivity below 0.1 μS/cm.
History
In order to eliminate or minimize the concentration polarization phenomenon present in electrodialysis systems, electrodeionization originated in the late 1950s. In 1956, William Katz at Ionics, developed one of the first Descriptions of electrodeionization and published his paper "The Present Status of Electric Membrane Demineralization" at the International Water Conference.
The technology was limited in application due to the low tolerance of hardness and organics. During the 1970s and 1980s reverse osmosis became a preferred technology to ion exchange resin for high TDS waters. As RO gained popularity, it was determined that EDI would be a suitable polishing technology. Packaged RO and EDI systems were used to displace chemically regenerated ion exchange systems.
In 1986 and 1989, companies like Millipore, Ionpure and Ionics Inc. developed electrodeionization devices. The initial devices were large, costly and often unreliable. In 1995 Glegg Water Conditioning introduced E-Cell brand electrodeionization. The new technology reduced cost and improve the reliability, based on a modular design standard. E-Cell was also offered to many OEMS and revolutionized the industry. Competitors soon followed with modular leak free designs.
Presently, this technology is widely available from many water treatment companies, but should only be applied by experts who understand the limitations and use top quality products.
Applications
When fed with low total dissolved solids (TDS) feed (e.g., feed purified by RO), the product can reach very high purity levels (e.g., [[Purified water#Electrical Conductivity|18 megohms/cm], Resistivity / Conductivity Measurement of Purified Water. The ion exchange resins act to retain the ions, allowing these to be transported across the ion exchange membranes. The main applications of EDI technology, such as that supplied by Ionpure, E-cell and SnowPure, are in electronics, pharmaceuticals and power generation.
Theory
An electrode in an electrochemical cell is referred to as either an anode or a cathode, terms that were coined by Michael Faraday. The anode is defined as the electrode at which electrons leave the cell and oxidation occurs, and the cathode as the electrode at which electrons enter the cell and reduction occurs. Each electrode may become either the anode or the cathode depending on the voltage applied to the cell. A bipolar electrode is an electrode that functions as the anode of one cell and the cathode of another cell.
Each cell consists of an electrode and an electrolyte with ions that undergo either oxidation or reduction. An electrolyte is a substance containing free ions that behaves as an electrically conductive medium. Because they generally consist of ions in solution, electrolytes are also known as ionic solutions, but molten electrolytes and solid electrolytes are also possible. They are sometimes referred to in abbreviated jargon as lytes.
Water is passed between an anode (positive electrode) and a cathode (negative electrode). Ion-selective membranes allow the positive ions to separate from the water toward the negative electrode and the negative ions toward the positive electrode. High purity deionized water results.
In situ regeneration
When using an excess of current that is higher that the necessary for the movement of the ions. A portion of the water will be split forming OH- and H+. This species will replace the anions and cations in the resin, this process is called regeneration in situ of the resin. And because it occurs during the process itself there is no need to stop the installation and use chemicals as it happens in others techniques.[1]
Installation scheme
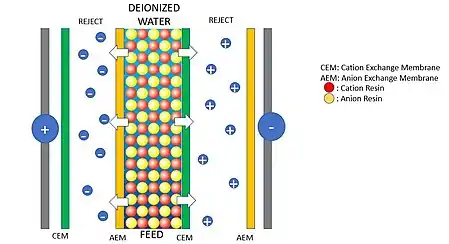
The typical EDI installation has the following components: anode and cathode, anion exchange membrane, cation exchange membrane and the resin. The most simplified configuration consist in 3 compartments, to increase the production these number can be increased.
The cations flow towards the cathode and the anions flows toward the anode. Only anions can go through the anion exchange membrane and only cations can go through the cation exchange membrane. This configuration allows anions and cations to only flow in one direction because of the membranes and the electric force, leaving the feed water free of ions, (deionized water).
The concentration flows (right and left of the feed flow) are rejected and they can be wasted, recycled, or use in another process.
The purpose of the ion exchange resin is to maintain stable conductance of the feed water. Without the resins, the conductance will drop dramatically as the concentration of ions is decreasing. Such drop off of conductance makes it very difficult to eliminate the 100% of ions. But using resins makes it possible.
References
- Alvarado, Lucía; Chen, Aicheng (2014-06-20). "Electrodeionization: Principles, Strategies and Applications". Electrochimica Acta. 132: 583–597. doi:10.1016/j.electacta.2014.03.165. ISSN 0013-4686.
External links
- video.
- Continuous Electrodeionization (CEDI/EDI) Ionpure CEDI Products
- CEDI University web site
- Electrodeionization (EDI), 1977 Inventors of EDI, SnowPure Water Technologies
- Electrodeionization (EDI), The Dow Chemical Company
- Electrodeionization Systems, Electrodeionization Systems
- Advanced Electrodeionization Technology for Product Desalting, Argonne National Laboratory