Electromeric effect
Electromeric effect refers to a molecular polarizability effect occurring by an intramolecular electron displacement (sometimes called the ‘conjugative mechanism’ and, previously, the ‘tautomeric mechanism’) characterized by the substitution of one electron pair for another within the same atomic octet of electrons.[1] The electromeric effect is often considered along with inductive effect as types of electron displacement.
Although some people refer it as an effect produced by the presence of a reagent like an electrophile or a nucleophile, IUPAC does not define it as such.[1]
The term electromeric effect is no longer used in standard texts and is considered as obsolete.[1] The concepts implied by the terms electromeric effect and mesomeric effect are absorbed in the term resonance effect.[2] This effect can be represented using curved arrows which symbolize the electron shift, as in the diagram below:
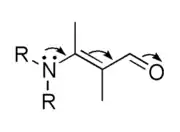
which represents this hypothetical electron shift:
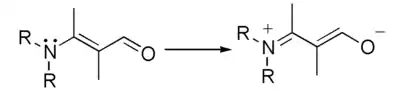
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "electromeric effect". doi:10.1351/goldbook.E01973
- https://goldbook.iupac.org/terms/view/M03844