Endoplasmic reticulum stress in beta cells
Beta cells are heavily engaged in the synthesis and secretion of insulin. They are therefore particularly sensitive to endoplasmic reticulum (ER) stress and the subsequent unfolded protein response (UPR). Severe or prolonged episodes of ER stress can lead to the death of beta cells,[1] which can contribute to the development of both Type I and Type II diabetes.[2]
ER stress in beta cells links obesity to type 2 diabetes and inflammation to type 1 diabetes.
ER stress in peripheral cells has also been linked to insulin resistance,[3] a precursor to type 2 diabetes.
Activation of ER stress
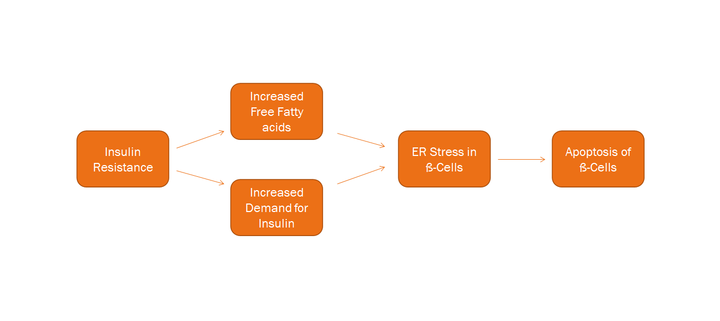
ER stress can be activated by a variety of factors. In experimental conditions, excessive lipid (which can happen following obesity, a common condition preceding type 2 diabetes) and pro-inflammatory cytokines (which can occur following an inflammation, a common cause for type 1 diabetes) can activate ER stress in beta cells.
Causes such as defective protein processing [4] and trafficking [5] or inappropriate calcium regulation [6] are likely in lipid-mediated ER stress. On the other hand, cytokines are likely to activate ER stress by decreasing the calcium pump Serca2b (also known as Atp2a2), leading to subsequent depletion in the ER calcium stores.[7]
ER Stress and Inflammation
All three of the pathways involved in the resolution of ER stress by the Unfolded Protein Response (UPR) are also related to inflammation.[8] The two pathways are very connected and both have been shown to activate each other[8]
Resolution of ER stress
Activation of ER stress by lipids results in a typical Unfolded Protein Response (UPR) to primarily restore ER function, whereas cytokine-activated ER stress leads to an atypical UPR that preferentially activate apoptosis in beta cells.[9]
The UPR is activated when GRP78, a.k.a. BiP, a protein-folding chaperone, is recruited to assist in protein folding. In ß-cells, insulin production is a major source of improperly folded protein.[1] GRP78 is normally bound to the luminal domain of ATF6, IRE1, and PERK, which prevents them from initiating their respective pathways of the UPR. When GRP78 is recruited to assist in protein folding, unbound ATF6, IRE1 and PERK are able to initiate the UPR.[10] The UPR is also activated by cytokines[8]
ER-stress activates Apoptosis through C/EBP Homologous Protein (CHOP)[11]
Measurement
ER Stress can be measured using Quantitative Real-Time RT-PCR to measure the rate at which XBP1 is cleaved by IRE1 when the UPR is activated.[12] XBP1 mRNA cleavage leads to the translation of a transcription factor for genes that resolve ER stress.[10] Measuring the rate at which XBP1 is cleaved gives a quantitative measure of ER stress in real time.
References
- Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42-61
- Laybutt DR, Preston AM, Akerfeldt MC et al (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50:752-763
- Wellen, Kathryn E.; Hotamisligil, Gökhan S. (2005-05-02). "Inflammation, stress, and diabetes". Journal of Clinical Investigation. 115 (5): 1111–1119. doi:10.1172/JCI25102. ISSN 0021-9738. PMC 1087185. PMID 15864338.
- Jeffrey KD, Alejandro EU, Luciani DS et al (2008) Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci USA 105:8452–8457
- Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ (2009) Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia 52:2369–2373
- Cunha DA, Hekerman P, Ladriere L et al (2008) Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci 121:2308–2318
- Oyadomari S, Takeda K, Takiguchi M et al (2001) Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98:10845–10850.
- Hotamisligil, Gökhan S. (March 2010). "Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease". Cell. 140 (6): 900–917. doi:10.1016/j.cell.2010.02.034. ISSN 0092-8674. PMC 2887297. PMID 20303879.
- Pirot P, Eizirik DL, Cardozo AK (2006) Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia 49:1229–1236
- Wang, Miao; Kaufman, Randal J. (January 2016). "Protein misfolding in the endoplasmic reticulum as a conduit to human disease". Nature. 529 (7586): 326–335. doi:10.1038/nature17041. ISSN 0028-0836.
- Hu, Hai; Tian, Mingxing; Ding, Chan; Yu, Shengqing (2019-01-04). "The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection". Frontiers in Immunology. 9: 3083. doi:10.3389/fimmu.2018.03083. ISSN 1664-3224. PMC 6328441. PMID 30662442.
- van Schadewijk, Annemarie; van’t Wout, Emily F. A.; Stolk, Jan; Hiemstra, Pieter S. (March 2012). "A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress". Cell Stress and Chaperones. 17 (2): 275–279. doi:10.1007/s12192-011-0306-2. ISSN 1355-8145. PMC 3273559. PMID 22038282.