Epargyreus clarus
Epargyreus clarus, the silver-spotted skipper, is a butterfly of the family Hesperiidae. It is claimed to be the most recognized skipper in North America.[2] E. clarus occurs in fields, gardens, and at forest edges and ranges from southern Canada throughout most of the United States to northern Mexico, but is absent in the Great Basin and western Texas.[3]
| Silver-spotted skipper | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Tribe: | |
| Genus: | |
| Species: | E. clarus |
| Binomial name | |
| Epargyreus clarus (Cramer, 1775) | |
 | |
E. clarus larvae create and reside in unique shelters stuck together with silk, which do not protect them from predators.[4] Natural predators of the species include paper wasp foragers, sphecid wasp and Crematogaster opuntiae ants.[5][6] The species is also considered to be a perching species, meaning that adult males compete for territory to attract females.[7] Although E. clarus is considered to be a pest of a few crop plants such as beans, its pest activity is not serious enough to warrant initiating major control measures.[5]
Geographic range
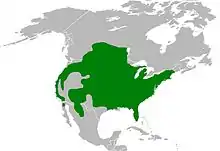
E. clarus has a wide distribution throughout North America: it ranges from southern Canada throughout most of the United States to northern Mexico, but is absent from the Great Basin and west Texas.[3]
Habitat
The silver-spotted skipper prefers open ranges where nectar plants are found, such as forest edges, swamps, brushy areas, and riparian habitats at lower elevations.[8][9]
Home range
At night, or on hot or cloudy days, E. clarus rests on the underside of a leaf. It hangs upside down, holding its wings together over its back so that its silver spot is exposed. E. clarus is often restricted by its habitat, preferring to fly in the shade.[8]
Food resources
Caterpillars
E. clarus caterpillars consume leaves of herbs, vines, shrubs, and trees in the pea family (Fabaceae) including false indigobush (Amorpha fruticosa), American hogpeanut (Amphicarpaea bracteata), groundnut (Apios americana), American wisteria (Wisteria frutescens), Atlantic pigeonwings or butterfly pea (Clitoria mariana), and the introduced Dixie ticktrefoil (Desmodium tortuosum), kudzu (Pueraria montana), black locust (Robinia pseudoacacia), Chinese wisteria (Wisteria sinensis) and an assortment of other legumes.[9]
Innate host plant preferences confer greater performance on larvae, due to differences in leaf nutrient concentrations. However, preference may be influenced by a larva’s rearing host species.[10]
Adults
Adults use their long tongues to feed on the nectar of a variety of flowers, mud, and even animal feces.[5] They almost never visit yellow flowers, favoring blue, red, pink, purple, and sometimes white and cream-colored ones. These include everlasting pea, common milkweed, red clover, buttonbush, blazing star, and thistles.[3]
Parental care
Oviposition
Females lay a single egg on or occasionally nearby host plants. They have been reported to oviposit on the least snoutbean (Rynchosia minima).[11]
Life cycle
Eggs
The eggs are green, with red coloration on top.[5]
Caterpillar

Larvae are yellow with transverse subdorsal black stripes. The first thoracic segment is black, has a brown prothoracic shield, and is significantly thinner than its adjacent organs – the head and second thoracic segment. The head is described as black or reddish brown with large with two prominent anterior orange spots, which mimic eyes. The three true legs and midabdominal true legs are all bright orange.[9][5]
Shelter building
All larval instars of E. clarus build shelters on their host plant. They build about 5 shelters throughout larval growth and development in 4 distinct styles, which are constructed from instinct and unique to larval size. The first, second, and third instars make a simpler, invariant structure that requires 2 incisions in the leaf and silk to fold over the flap created. Shelters are typically built on the apical portion of the leaflet. One study of E. clarus larvae in the Washington D.C. area defined 5 steps of shelter building:[4]
- Site selection: Larvae traverse across the entire leaf, following major veins and swinging their heads to create a single silk trail.
- Silk template: A thick, visible mat of silk outlines the intended incisions and ultimately becomes the ceiling of the leaf house. The larvae keep their posterior end on the template at all times.
- First cut: The first cut follows the silk template on the perimeter nearest the leaf apex. The larvae often interrupt chewing cuts in order to deposit silk at the terminus. The first cut is completed in about 6-7 segments. At the end, the larva makes a U-turn motion so that its position relative to the leaf surface and template margins remains the same while facing the opposite direction.
- Second cut and notch: The second cut follows the silk template on the edge closest to the base of the leaflet and is completed in about 2 to 4 segments. The first segment curves inwards, is made in a single effort, and is about 75% the length of the first cut. The next segment is adjacent to the initial segment and continues past the length of the first cut. The juncture between the two segments creates a notch.
- Folding and securing: The uncut area between the first and second incisions composes the hinge. The hinge length tends to correspond to larval body length. The larva lay silk strands along this area, parallel to the cut termini. As the strands contract, the notch creates a pinch and the leaf flap is pulled over the leaflet plane. The pinch then becomes the peak of the roof and the larva begins to make “guy-wires” that attach the edge of the flap to the leaflet surface.
Larvae take breaks of about 30 minutes between each cut and fold. It typically takes less than 2 and a half hours to complete shelter construction. Larvae spend about 95% of their time resting on the ceiling of the shelter.[4]

First instar shelters are typically fasted by only about 2-4 “guy-wires.” In addition, older caterpillars occasionally live in a nest of made of multiple leaves connected by silk, especially when using host plants with smaller leaflets. For example, first, second, and third instars can make shelters with one Robinia leaflet, but the fourth and fifth instars have to use 2 to 4 or 5 leaflets.[4]
Pupa
Larvae pupate throughout the winter. The pupa is dark brown with black and white marks. There are three or more overlapping generations of hibernating pupa in Florida – fewer in the northern regions.[5]
Adult
The adult wingspan ranges from 1.75 to 2.625 inches (4.5 to 6.7 cm). Each forewing is triangular and dark brown with a large yellow-orange medial patch. Male forewings are pointed than those of females. The hindwings are also dark brown with a large silver patch on the discal third of the ventral side.[9][5]
Migration
Adults are diurnal and fly from early to midsummer.[9] They have one brood per year in the North and West, two in the East, and three to four in the Deep South.[3]
Enemies
Predators
Common predators include the paper wasp foragers Polistes fuscatus and Polistes dominula, as well as Crematogaster opuntiae ants. Studies have found that shelters protect larvae from these predators in lab settings. Shelters delayed or prevented Crematogaster opuntiae colonies from detecting the larvae. This is likely because of these ants’ limited senses, in conjunction with the inhibitory structure of the shelter: they are only able to detect a motionless caterpillar within approximately one body length, and this is further obstructed by the mere single small opening into the shelter. While wasps in the laboratory setting did spend more time on areas of the leaf damaged by feeding or silk deposition, the larval shelter prevented visual detection and posed a physical barrier. However, shelters did not protect against these predators in the field. Wasps only spend a few seconds on a leaflet to successfully identify and remove the larva from its shelter. This suggests that shelter identification and larval extraction is a learned ability, perhaps developed through visual or olfactory cues.[6]
Other predators include the sphecid wasp Stictia carolina, which sometimes supplies its nests with silver-spotted skipper larvae. Other predators are also often attracted to the chemical cues from frass, or insect excrement. E. clarus larvae use an anal comb to throw their frass up to 38 body lengths away from them. When disturbed, larvae regurgitate an unpleasant, greenish chemical in defense.[5]
Parasites
E. clarus larvae are also a natural host of Baculovirus. The advanced stages of this infection cause larvae to feed without resting. Later, they climb to higher and more exposed areas, where they are more conspicuous to predators. Predator attack leaves their infected remains to be absorbed by the surrounding vegetation, which will be consumed by new hosts.[5]
Mating
Instead of receiving ejaculate, a female receives a large spermatophore from a male for each copulation. Thus, the number of spermatophores a female contains roughly equates to the number of copulations. Generally, when there are more than two spermatophores present in a female, each one’s placement and degree of erosion indicates its age relative to the other. E. clarus, however, is particularly resistant to erosion. Once mated, females seem to reject males, at least for some time afterwards.[12]
Male defense of places likely to attract females
Males perch on branches and tall weeds about 1–2 m above the ground, darting out when any insect passes in the hope for a receptive E. clarus female. When confronted by another conspecific, male movements are swift and acrobatic. This perching behavior is part of a common mate-location strategy, in which males compete for sites where females are more likely to occur.[7] Females do not engage in this behavior, but may have to fly considerable distances to find mates depending on the proximity of male territory to larval development site. Such sites are roughly the size of a small room and are most likely chosen based on vegetation and topography.[8]
Physiology
Vision
E. clarus possesses compound eyes that lack pigment in the iris region. Each ommatidia, or single optical unit, has its own unique visual field that spans about 2°. The small visual field can be due to crystalline tracts in the eye that restrict the light to reach the retina only through this path.[13]
Interaction with plants
.jpg.webp)
E. clarus is known to be a nectar-thief species. An individual nectar thief is an animal that takes nectar from a flower but does not pollinate it while doing so. A species as a whole can be considered a thief species if it does not pollinate a species of flowers during more than 50% of its feedings. Research has found that while foraging on flowers, the butterfly tended to probe the innermost disk florets, which are the male organs, but not make contact with the outermost florets, which are the female organs.[14]
Pest of crop plants
E. clarus is known to feed on various crop plants such as soybean and kidney bean. However, no large-scale control measures have been taken as the species' pest activity is not too detrimental.[5]
References
- "NatureServe Explorer 2.0 Epargyreus clarus Silver-spotted Skipper". explorer.natureserve.org. Retrieved 1 October 2020.
- Kaufman, Kenn; Eaton, Eric R. (2007). Kaufman Field Guide to Insects of North America. Houghton Mifflin Books. pp. 236–237. ISBN 978-0-618-15310-7.
- "Silver-spotted skipper". Butterflies and Moths of North America. Archived from the original on 2009-01-16. Retrieved 2008-10-11.
- Weiss, Martha R.; Lind, Eric M.; Jones, Meg T.; Long, Jeremy D.; Maupin, Jennifer L. (2003-07-01). "Uniformity of Leaf Shelter Construction by Larvae of Epargyreus clarus (Hesperiidae), the Silver-Spotted Skipper". Journal of Insect Behavior. 16 (4): 465–480. doi:10.1023/A:1027399122333. ISSN 0892-7553. S2CID 28669353.
- Hall, Donald W. (2017). "Silver-Spotted Skipper Epargyreus clarus (Cramer)". The Institute of Food and Agricultural Sciences Extension.
- Jones, Meg T.; Castellanos, Ignacio; Weiss, Martha R. (2002-12-01). "Do leaf shelters always protect caterpillars from invertebrate predators?". Ecological Entomology. 27 (6): 753–757. doi:10.1046/j.1365-2311.2002.00465.x. ISSN 1365-2311. S2CID 86245610.
- Scott, James A. (1974). "Mate-Locating Behavior of Butterflies". The American Midland Naturalist. 91 (1): 103–117. doi:10.2307/2424514. JSTOR 2424514.
- Shapiro, Arthur M. (1978). "Phenotypic and Behavioral Convergence of "Silver-Spotted Skippers" (Lepidoptera: Hesperiidae)". Biotropica. 10 (2): 159–160. doi:10.2307/2388021. JSTOR 2388021.
- Miller, Jeffrey C.; Hammond, Paul C. (2003). LEPIDOPTERA OF THE PACIFIC NORTHWEST: CATERPILLARS AND ADULTS. p. 28.
- Rosenwald, Laura C.; Lill, John T.; Lind, Eric M.; Weiss, Martha R. (2017-05-10). "Dynamics of host plant selection and host-switching by silver-spotted skipper caterpillars". Arthropod-Plant Interactions. 11 (6): 833–842. doi:10.1007/s11829-017-9538-0. ISSN 1872-8855. S2CID 37474805.
- Scott, JA (1986). The Butterflies of North America: A Natural History and Field Guide. Stanford University Press. p. 583.
- Burns, John M. (1968). "Mating Frequency in Natural Populations of Skippers and Butterflies as Determined by Spermatophore Counts". Proceedings of the National Academy of Sciences of the United States of America. 61 (3): 852–859. doi:10.1073/pnas.61.3.852. JSTOR 58962. PMC 305406. PMID 16591715.
- Doving, K. B. (1969). "Function of Insect Compound Eyes Containing Crystalline Tracts". The Journal of General Physiology. 54 (2): 250–267. CiteSeerX 10.1.1.282.9479. doi:10.1085/jgp.54.2.250. PMC 2225925. PMID 4894303.
- Venables, Adrienne; Barrows, Edward (1985). "SKIPPERS: POLLINATORS OR NECTAR THIEVES?" (PDF). Journal of the Lepidopterists' Society. 39 (4): 299–312.
- Darby, Gene (1958). What is a Butterfly. Chicago: Benefic Press. p. 27.
- Evans, Arthur V. (2007). "Butterflies and Moths:Order Lepidoptera". Field Guide to Insects and Spiders of North America. Sterling Publishing. p. 304. ISBN 978-1-4027-4153-1.
- Kaufman, Kenn; Brock, Jim P. (2003). Kaufman Field Guide to Butterflies of North America. Houghton Mifflin Books. pp. 256–257. ISBN 978-0-618-76826-4.
External links
| Wikimedia Commons has media related to Epargyreus clarus. |
- Epargyreus clarus, silver-spotted skipper on the UF / IFAS Featured Creatures Web site