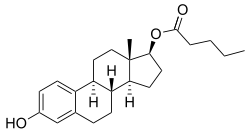
Estradiol valerate
Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. In women, it is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender women, and in hormonal birth control.[4][3][10][11] It is also used in the treatment of prostate cancer in men.[10] The medication is taken by mouth or by injection into muscle once every 1 to 4 weeks.[10][11]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛstrəˈdaɪoʊl ˈvæləreɪt/ ES-trə-DY-ohl VAL-ə-rayt[1] |
| Trade names | Delestrogen, Progynon Depot, Progynova, many others |
| Other names | EV; E2V; Oestradiol valerate; Estradiol pentanoate; Estradiol valerianate |
| Routes of administration | By mouth, sublingual, intramuscular injection,[2] subcutaneous injection |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 3–5%[3][4] IM injection: 100%[3] |
| Protein binding | Estradiol: ~98% (to albumin and SHBG)[5][6] |
| Metabolism | Cleavage via esterases in the liver, blood, and tissues[3] |
| Metabolites | Estradiol, valeric acid, and metabolites of estradiol[3] |
| Elimination half-life | Oral: 12–20 hours (as E2)[3][5] IM injection: 4–5 days[3] |
| Duration of action | IM injection: • 5 mg: 7–8 days[7] • 10 mg: 10–14 days[8][9] • 40 mg: 2–3 weeks[8] • 100 mg: 3–4 weeks[8] |
| Excretion | Urine (80%)[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.327 |
| Chemical and physical data | |
| Formula | C23H32O3 |
| Molar mass | 356.498 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 144 to 145 °C (291 to 293 °F) |
| |
| |
Side effects of estradiol valerate include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[12][10][11] Estradiol valerate is a synthetic estrogen and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[4][3][13] It is an estrogen ester and a prodrug of estradiol in the body.[13][4][3] Because of this, it is considered to be a natural and bioidentical form of estrogen.[13][14][3][15]
Estradiol valerate was first described in 1940 and was introduced for medical use in 1954.[16][17][18] Along with estradiol cypionate, it is one of the most widely used esters of estradiol.[19] Estradiol valerate is used in the United States, Canada, Europe, and throughout much of the rest of the world.[20][21] It is available as a generic medication.[22]
Medical uses
The medical uses of estradiol valerate are the same as those of estradiol and other estrogens. Examples of indications for the medication include hormone therapy and hormonal contraception. In regard to the latter, estradiol valerate is available in combination with a progestin as a combined estradiol-containing oral contraceptive (with dienogest)[23] and as a combined injectable contraceptive.[24][25][26] Along with estradiol cypionate, estradiol undecylate, and estradiol benzoate, estradiol valerate is used as a form of high-dose estrogen therapy in feminizing hormone therapy for transgender women.[27][28][29][30] It is also used as a form of high-dose estrogen therapy in the treatment of prostate cancer in men.[10] Low-dose oral estradiol valerate (2–6 mg/day) has been used in the treatment of breast cancer in women who were previously treated with and benefited from but acquired resistance to aromatase inhibitors as well.[31][32]
In the United States, the approved indications of estradiol valerate injections include the treatment of moderate to severe hot flashes and vaginal atrophy associated with menopause in women, the treatment of hypoestrogenism due to hypogonadism, castration, or primary ovarian failure in women, and the palliative treatment of advanced prostate cancer in men.[10] Elsewhere in the world, oral estradiol valerate is similarly approved for the treatment of symptoms associated with menopause or hypoestrogenism due to castration in women.[11] Such symptoms may include hot flashes, outbreaks of sweat, sleep disturbances, depressive moods, irritability, headaches, and dizziness.[11]
Estradiol valerate by intramuscular injection is usually used at a dosage of 10 to 20 mg every 4 weeks in the treatment of menopausal symptoms and hypoestrogenism due to hypogonadism, castration, or primary ovarian failure in women.[10] In the past, it was used at even higher doses of 10 to 40 every 1 to 4 weeks for estrogen replacement.[33] Estradiol valerate is usually used in the treatment of advanced prostate cancer in men at a dosage of 30 mg or more every 1 to 2 weeks by intramuscular injection.[10] In transgender women, estradiol valerate given by intramuscular injection is usually used at a dosage of 5 to 20 mg, but up to 30 to 40 mg, once every 2 weeks.[28][29][27] Estradiol valerate has also been used at a dose of 10 to 40 mg by intramuscular injection to limit bleeding in women with hemorrhage due to dysfunctional uterine bleeding.[34]:318[35]:60
| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | 2.5 μg/day | 5–15 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
| Transdermal gel | Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
| Vaginal | Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
| IM or SC injection | Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
Available forms
Estradiol valerate is and has been available in the form of vials and ampoules of oil solution for intramuscular injection in concentrations of 4, 5, 10, 20, and 40 mg/mL and in the form of oral tablets at doses of 0.5, 1, 2, and 4 mg per tablet.[36][16][37][38] In the United States, it is specifically available in formulations of 10, 20, and 40 mg/mL in oil solution (as Delestrogen, as well as generics).[36] Aside from estradiol valerate, the only other injectable estrogen formulations that remain available in the United States are estradiol cypionate (5 mg/mL in oil solution) and conjugated estrogens (25 mg/vial in solution).[36] Some or all oral estradiol valerate tablets are micronized, similarly to oral estradiol tablets.[39]
In addition to single-drug formulations, oral estradiol valerate is available in combination with the progestin dienogest as a combined oral contraceptive and intramuscular estradiol valerate is marketed at a concentration of 5 mg/mL in combination with the progestin hydroxyprogesterone caproate and with the progestin norethisterone enantate as combined injectable contraceptives.[36][23][24][25][26][1] Intramuscular estradiol valerate is also marketed at a concentration of 4 mg/mL in combination with the weak androgen and neurosteroid prasterone enanthate (DHEA enanthate) and with the androgen testosterone enantate for use in menopausal hormone therapy, but the latter formulation has been discontinued.[40][36] The availability of estradiol valerate-containing products varies throughout the world.[1]
| Route | Ingredient | Form | Dose[lower-alpha 2] | Brand names[lower-alpha 3] |
|---|---|---|---|---|
| Oral | Estradiol | Tablet | 0.1, 0.2, 0.5, 1, 2, 4 mg | Estrace, Ovocyclin |
| Estradiol valerate | Tablet | 0.5, 1, 2, 4 mg | Progynova | |
| Transdermal | Estradiol | Patch | 14, 25, 37.5, 50, 60, 75, 100 µg/d | Climara, Vivelle |
| Gel pump | 0.06% (0.52, 0.75 mg/pump) | Elestrin, EstroGel | ||
| Gel packet | 0.1% (0.25, 0.5, 1.0 mg/pk.) | DiviGel, Sandrena | ||
| Emulsion | 0.25% (25 µg/pouch) | Estrasorb | ||
| Spray | 1.53 mg/spray | Evamist, Lenzetto | ||
| Vaginal | Estradiol | Tablet | 10, 25 µg | Vagifem |
| Cream | 0.01% (0.1 mg/gram) | Estrace | ||
| Insert | 4, 10 µg | Imvexxy | ||
| Ring | 2 mg/ring (7.5 µg/d, 3 mon.) | Estring | ||
| Estradiol acetate | Ring | 50, 100 µg/d, 3 months | Femring | |
| Injection[lower-alpha 4] | Estradiol | Microspheres | 1 mg/mL | Juvenum E |
| Estradiol benzoate | Oil solution | 0.167, 0.2, 0.333, 1, 1.67, 2, 5, 10, 20, 25 mg/mL | Progynon-B | |
| Estradiol cypionate | Oil solution | 1, 3, 5 mg/mL | Depo-Estradiol | |
| Estradiol valerate | Oil solution | 5, 10, 20, 40 mg/mL | Progynon Depot | |
| Implant | Estradiol | Pellet | 20, 25, 50, 100 mg, 6 mon. | Estradiol Implants |
Notes and sources:
| ||||
Contraindications
Contraindications of estrogens include coagulation problems, cardiovascular diseases, liver disease, and certain hormone-sensitive cancers such as breast cancer and endometrial cancer, among others.[54][55][56][57]
Side effects
The side effects of estradiol valerate are the same as those of estradiol. Examples of such side effects include breast tenderness and enlargement, nausea, bloating, edema, headache, and melasma.[12][58] High-dose estrogen therapy with estradiol valerate injections may also cause an increased risk of thromboembolism, changes in blood lipid profile, increased insulin resistance, and increased levels of prolactin.[58]
Overdose
Estradiol valerate has been used at very high doses of 40 to 100 mg once per week in women and men, without overt signs of acute toxicity observed.[59][60][61][62][63][64][65][66][67][68][69] Symptoms of estrogen overdosage may include nausea, vomiting, bloating, increased weight, water retention, breast tenderness, vaginal discharge, heavy legs, and leg cramps.[54] These side effects can be diminished by reducing the estrogen dosage.[54]
Interactions
Inhibitors and inducers of cytochrome P450 may influence the metabolism of estradiol and by extension circulating estradiol levels.[70]
Pharmacology
Pharmacodynamics
Estradiol valerate is an estradiol ester, or a prodrug of estradiol.[13][4] As such, it is an estrogen, or an agonist of the estrogen receptors.[4][13] The affinity of estradiol valerate for the estrogen receptor is approximately 50 times lower than that of estradiol.[3] In addition, estradiol valerate is rapidly cleaved into estradiol and is unable to reach target tissues in concentrations of significance, if at all.[3] As such, estradiol valerate is essentially inactive in terms of estrogenic effect itself, acting solely as a prodrug to estradiol.[3] The molecular weight of estradiol valerate is about 131% of that of estradiol due to the presence of its C17β valerate ester, and hence estradiol valerate contains about 76% of the amount of estradiol of an equal dose of estradiol.[20][21] Aside from dose adjustment to account for the difference in molecular weight, oral estradiol valerate is considered to be equivalent to oral estradiol.[3] Because estradiol valerate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[13][14][15]
| Estrogen | Other names | RBA (%)a | REP (%)b | |||
|---|---|---|---|---|---|---|
| ER | ERα | ERβ | ||||
| Estradiol | E2 | 100 | 100 | 100 | ||
| Estradiol 3-sulfate | E2S; E2-3S | ? | 0.02 | 0.04 | ||
| Estradiol 3-glucuronide | E2-3G | ? | 0.02 | 0.09 | ||
| Estradiol 17β-glucuronide | E2-17G | ? | 0.002 | 0.0002 | ||
| Estradiol benzoate | EB; Estradiol 3-benzoate | 10 | 1.1 | 0.52 | ||
| Estradiol 17β-acetate | E2-17A | 31–45 | 24 | ? | ||
| Estradiol diacetate | EDA; Estradiol 3,17β-diacetate | ? | 0.79 | ? | ||
| Estradiol propionate | EP; Estradiol 17β-propionate | 19–26 | 2.6 | ? | ||
| Estradiol valerate | EV; Estradiol 17β-valerate | 2–11 | 0.04–21 | ? | ||
| Estradiol cypionate | EC; Estradiol 17β-cypionate | ?c | 4.0 | ? | ||
| Estradiol palmitate | Estradiol 17β-palmitate | 0 | ? | ? | ||
| Estradiol stearate | Estradiol 17β-stearate | 0 | ? | ? | ||
| Estrone | E1; 17-Ketoestradiol | 11 | 5.3–38 | 14 | ||
| Estrone sulfate | E1S; Estrone 3-sulfate | 2 | 0.004 | 0.002 | ||
| Estrone glucuronide | E1G; Estrone 3-glucuronide | ? | <0.001 | 0.0006 | ||
| Ethinylestradiol | EE; 17α-Ethynylestradiol | 100 | 17–150 | 129 | ||
| Mestranol | EE 3-methyl ether | 1 | 1.3–8.2 | 0.16 | ||
| Quinestrol | EE 3-cyclopentyl ether | ? | 0.37 | ? | ||
| Footnotes: a = Relative binding affinities (RBAs) were determined via in-vitro displacement of labeled estradiol from estrogen receptors (ERs) generally of rodent uterine cytosol. Estrogen esters are variably hydrolyzed into estrogens in these systems (shorter ester chain length -> greater rate of hydrolysis) and the ER RBAs of the esters decrease strongly when hydrolysis is prevented. b = Relative estrogenic potencies (REPs) were calculated from half-maximal effective concentrations (EC50) that were determined via in-vitro β‐galactosidase (β-gal) and green fluorescent protein (GFP) production assays in yeast expressing human ERα and human ERβ. Both mammalian cells and yeast have the capacity to hydrolyze estrogen esters. c = The affinities of estradiol cypionate for the ERs are similar to those of estradiol valerate and estradiol benzoate (figure). Sources: See template page. | ||||||
| Compound | Dosage for specific uses (mg usually)[lower-alpha 1] | ||||||
|---|---|---|---|---|---|---|---|
| ETD[lower-alpha 2] | EPD[lower-alpha 2] | MSD[lower-alpha 2] | MSD[lower-alpha 3] | OID[lower-alpha 3] | TSD[lower-alpha 3] | ||
| Estradiol (non-micron.) | 30 | ≥120–300 | 120 | 6 | - | - | |
| Estradiol (micronized) | 6–12 | 60–80 | 14–42 | 1–2 | >5 | >8 | |
| Estradiol valerate | 6–12 | 60–80 | 14–42 | 1–2 | - | >8 | |
| Estradiol benzoate | - | 60–140 | - | - | - | - | |
| Estriol | ≥20 | 120–150[lower-alpha 4] | 28–126 | 1–6 | >5 | - | |
| Estriol succinate | - | 140–150[lower-alpha 4] | 28–126 | 2–6 | - | - | |
| Estrone sulfate | 12 | 60 | 42 | 2 | - | - | |
| Conjugated estrogens | 5–12 | 60–80 | 8.4–25 | 0.625–1.25 | >3.75 | 7.5 | |
| Ethinylestradiol | 200 μg | 1–2 | 280 μg | 20–40 μg | 100 μg | 100 μg | |
| Mestranol | 300 μg | 1.5–3.0 | 300–600 μg | 25–30 μg | >80 μg | - | |
| Quinestrol | 300 μg | 2–4 | 500 μg | 25–50 μg | - | - | |
| Methylestradiol | - | 2 | - | - | - | - | |
| Diethylstilbestrol | 2.5 | 20–30 | 11 | 0.5–2.0 | >5 | 3 | |
| DES dipropionate | - | 15–30 | - | - | - | - | |
| Dienestrol | 5 | 30–40 | 42 | 0.5–4.0 | - | - | |
| Dienestrol diacetate | 3–5 | 30–60 | - | - | - | - | |
| Hexestrol | - | 70–110 | - | - | - | - | |
| Chlorotrianisene | - | >100 | - | - | >48 | - | |
| Methallenestril | - | 400 | - | - | - | - | |
| Estrogen | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | ? | ? | ? | 0.3 | 0.3 | ? | ? | ? | ? | ? |
| Estriol | 0.3 | 0.3 | 0.1 | 0.3 | 0.3 | 0.2 | ? | ? | ? | 0.67 |
| Estrone sulfate | ? | 0.9 | 0.9 | 0.8–0.9 | 0.9 | 0.5 | 0.9 | 0.5–0.7 | 1.4–1.5 | 0.56–1.7 |
| Conjugated estrogens | 1.2 | 1.5 | 2.0 | 1.1–1.3 | 1.0 | 1.5 | 3.0–3.2 | 1.3–1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | ? | ? | 1.0 | ? | ? | 6.0 | 7.5 | 6.0 | 7.5 | ? |
| Ethinylestradiol | 120 | 150 | 400 | 60–150 | 100 | 400 | 500–600 | 500–600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | ? | ? | ? | 2.9–3.4 | ? | ? | 26–28 | 25–37 | 20 | 5.7–7.5 |
Sources and footnotes
Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these liver proteins. Liver = Ratio of liver estrogenic effects to general/systemic estrogenic effects (hot flashes/gonadotropins). Sources: See template. | ||||||||||
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Effects on liver protein synthesis
The influence of 2 mg/day oral estradiol valerate on coagulation factors is less than that of 10 μg/day oral ethinylestradiol.[90][23][91][92][93] Oral ethinylestradiol at 10 μg/day has been found to have about 1.5- to 2.5-fold the impact of 2 mg/day oral estradiol valerate on HDL cholesterol and triglycerides.[94][95][96] The influence of 20 or 50 μg/day oral ethinylestradiol on coagulation factors and HDL cholesterol is markedly greater than that of 2 mg/day oral estradiol valerate.[94][97]
Estradiol-containing birth control pills, which contain 1 to 3 mg/day estradiol or estradiol valerate, have been found to increase sex hormone-binding globulin (SHBG) levels by 1.5-fold.[98][99] Oral estradiol valerate at 6 mg/day has been found to increase SHBG levels by 2.5- to 3-fold in transgender women.[100][101] For comparison, combined birth control pills containing ethinylestradiol and a progestin with minimal androgenic or antiandrogenic activity have been found to increase SHBG levels by about 3- to 4-fold.[102]
Pharmacokinetics
Regardless of the route of administration, estradiol valerate behaves as a prodrug of estradiol via cleavage by esterases into estradiol and the natural fatty acid valeric acid.[4][13][3][103] This cleavage occurs not only in the liver, but also in the blood and in tissues, and the hydrolysis of estradiol valerate into estradiol and valeric acid is complete regardless of whether the medication is administered orally or parenterally.[3] High levels of circulating estradiol are found after an intravenous injection of estradiol valerate, and this indicates very rapid cleavage of the medication upon entering circulation.[3]
Oral administration
Esterification of the C17β position of estradiol as in estradiol valerate reduces the metabolism of estradiol valerate by 17β-hydroxysteroid dehydrogenase (17β-HSD).[4] As approximately 80% of estradiol is metabolized into estrone (and estrone sulfate) by 17β-HSD during first-pass metabolism, this improves the metabolic stability and hence bioavailability of estradiol valerate.[13] However, estradiol valerate is hydrolyzed into estradiol and valeric acid in the intestines, and hence, is still subject to extensive first-pass metabolism.[4] As such, the oral bioavailability of estradiol valerate is only around 3 to 5%, and is similar to that of oral estradiol.[3][4][104] All oral tablets in the cases of both estradiol and estradiol valerate seem to be micronized.[39] Due to its nature as a rapidly converted prodrug of estradiol, the pharmacokinetics of oral estradiol valerate are similar to those of oral estradiol.[3][4] Moreover, the pharmacodynamics and potency (after differences in molecular weight are taken into account) of oral estradiol valerate are considered to be equivalent to those of oral estradiol.[3] This is also notably true for effects on hepatic protein synthesis (e.g., of SHBG), again after differences in molecular weight between the two compounds are considered.[3]
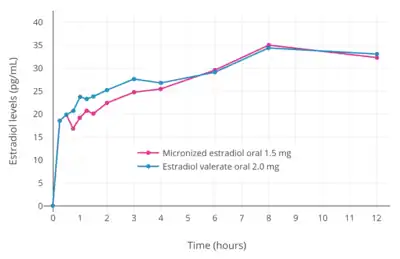
A dosage of 1 mg/day oral estradiol valerate has been found to produce approximate circulating concentrations of 50 pg/mL estradiol and 160 pg/mL estrone, while a dosage of 2 mg/day results in circulating levels of 60 pg/mL estradiol and 300 pg/mL estrone.[105] These concentrations of estradiol and estrone are comparable to those observed with 1 and 2 mg/day oral estradiol.[105] A review of selected studies reported a range of mean peak estradiol levels of 24 to 140 pg/mL occurring 1 to 12 hours after administration of 2 mg oral estradiol valerate.[3] A study found that, in accordance with their differences in molecular weights, oral estradiol produced higher levels of estradiol than oral estradiol valerate.[106] Likewise, other studies found that levels of estradiol and estrone are very similar after oral administration of roughly equimolar doses of estradiol (1.5 mg) and estradiol valerate (2 mg).[107][108][109] A study of high-dose oral estradiol valerate found levels of estradiol of about 250 pg/mL after a single 10-mg dose in three women.[104]
- Hormone levels with oral estradiol valerate
Sublingual administration
_taken_sublingually_in_premenopausal_women.png.webp)
Estradiol valerate has been studied by sublingual administration in premenopausal women for the purpose of cycle control and ovulation suppression in egg donation and surrogacy.[111][112] It has been investigated for this indication, along with vaginal and transdermal estradiol, because oral estradiol valerate is sometimes unable to achieve adequate estradiol levels and hence proper cycle control in this situation.[111][112] Sublingual administration of estradiol valerate bypasses the first pass that occurs with the oral route and results in higher levels of estradiol and improved cycle control.[111][112] Sublingual estradiol valerate is also used in hormone therapy for transgender women.[113]
The administration of 2 mg oral micronized estradiol valerate tablets (Progynova, Schering) sublingually 3 or 4 times per day has been found to result in circulating estradiol levels of about 290 pg/mL to 460 pg/mL in premenopausal women (time of measurements not given).[111][112] Steady-state levels of estradiol were achieved within about 2 or 3 days.[111][112] Levels of progesterone, luteinizing hormone, and follicle-stimulating hormone were all considerably suppressed, and ovulation, as well as the associated mid-cycle hormonal surges, were prevented.[111][112] Similarly to oral administration of estradiol, but in contrast to the vaginal and transdermal routes, the ratio of estradiol to estrone is decreased with sublingual administration of either estradiol valerate or estradiol.[111][112][114]
Intramuscular injection
In contrast to oral administration, the bioavailability of estradiol valerate is complete (i.e., 100%) via intramuscular injection.[3][4] Due to the far greater bioavailability of intramuscular estradiol valerate relative to oral, the former is substantially stronger (in terms of potency) than the latter.[3] As an example, a single 4 mg intramuscular injection is said to be approximately equivalent to 2 mg/day of the medication administered orally over the course of 3 weeks.[3] Estradiol valerate, when given intramuscularly in oil, has a relatively long duration due to the formation of an intramuscular depot from which the medication is slowly released and absorbed.[3][115] Upon intramuscular injection of estradiol valerate in an oil solution, the solvent (i.e., oil) is absorbed, and a primary microcrystalline depot is formed within the muscle at the site of injection.[4] In addition, a secondary depot may also be formed in adipose tissue.[4] The slow release of estradiol valerate is caused by the increased lipophilicity of the medication, which in turn is due to its long fatty acid valeric acid ester moiety.[3] The elimination half-life of intramuscularly administered estradiol valerate in oil is reported to be 4 to 5 days.[3]
A single intramuscular injection of 4 mg estradiol valerate has been found to result in maximal circulating levels of estradiol of about 390 pg/mL within 3 days of administration, with levels declining to 100 pg/mL (baseline, in the study) by 12 to 13 days.[40] Studies in general have found that a single intramuscular injection of 4 mg estradiol valerate results in peak levels of estradiol of 240 to 540 pg/mL after 1 to 5 days following administration.[116] A study found that a single intramuscular injection of 5 mg estradiol valerate resulted in peak circulating levels of 667 pg/mL estradiol and 324 pg/mL estrone within approximately 2 and 3 days, respectively.[7] The duration of estradiol valerate at this dose and in this study was considered to be 7 to 8 days.[7] Other studies have found that larger doses of intramuscular estradiol valerate exceeding 20 mg have a duration of more than 15 days.[7] A third study, in contrast to the preceding study, found that a single 10 mg intramuscular injection of estradiol valerate resulted in maximal estradiol levels of 506 to 544 pg/mL and maximal estrone levels of 205 to 219 pg/mL in postmenopausal women.[117]
With intramuscular injections of estradiol valerate, it has been reported that a dose of 5 mg has a duration of 7 to 8 days, 10 mg a duration of 10 to 14 days, 40 mg a duration of 2 to 3 weeks (14 to 21 days), and 100 mg a duration of 3 to 4 weeks (21 to 28 days).[8][9][7]
A study of pseudopregnancy with intramuscular injections of 40 mg/week estradiol valerate and 250 mg/week hydroxyprogesterone caproate in women with estrogen deficiency observed estradiol levels of about 3,100 pg/mL at 3 months of therapy and 2,500 pg/mL at 6 months of therapy.[60]
| Estrogen | Dose | Peak levels | Time to peak | Duration |
|---|---|---|---|---|
| Estradiol benzoate | 5 mg | E2: 940 pg/mL E1: 343 pg/mL | E2: 1.8 days E1: 2.4 days | 4–5 days |
| Estradiol valerate | 5 mg | E2: 667 pg/mL E1: 324 pg/mL | E2: 2.2 days E1: 2.7 days | 7–8 days |
| Estradiol cypionate | 5 mg | E2: 338 pg/mL E1: 145 pg/mL | E2: 3.9 days E1: 5.1 days | 11 days |
| Notes: All via i.m. injection of oil solution. Determinations via radioimmunoassay with chromatographic separation. Sources: See template. | ||||
- Hormone levels with estradiol valerate by intramuscular injection
 Estrogen levels after a single intramuscular injection of 10 mg estradiol valerate in oil in 24 postmenopausal women.[117] Determinations were made for both Progynon Depot 10 and Estradiol Depot 10, for a total of 48 measurements per point.[117] Assays were performed using GC/MS-NCI/SIM.[117] Source was Schug et al. (2012).[117]
Estrogen levels after a single intramuscular injection of 10 mg estradiol valerate in oil in 24 postmenopausal women.[117] Determinations were made for both Progynon Depot 10 and Estradiol Depot 10, for a total of 48 measurements per point.[117] Assays were performed using GC/MS-NCI/SIM.[117] Source was Schug et al. (2012).[117] Hormone levels after a single intramuscular injection of 5 mg estradiol valerate in oil in 17 postmenopausal women.[118] Assays were performed using EIA.[118] Estrone levels were likely overestimated, possibly due to cross reactivity of the assay with estrogen conjugates.[117] Source was Göretzlehner et al. (2002).[118]
Hormone levels after a single intramuscular injection of 5 mg estradiol valerate in oil in 17 postmenopausal women.[118] Assays were performed using EIA.[118] Estrone levels were likely overestimated, possibly due to cross reactivity of the assay with estrogen conjugates.[117] Source was Göretzlehner et al. (2002).[118]
 Estradiol levels after a single intramuscular injection of 10 mg estradiol valerate or 100 mg estradiol undecylate in oil both in 4 individuals each.[119] Subject characteristics and assay method were not described.[119] Source was Vermeulen (1975).[119]
Estradiol levels after a single intramuscular injection of 10 mg estradiol valerate or 100 mg estradiol undecylate in oil both in 4 individuals each.[119] Subject characteristics and assay method were not described.[119] Source was Vermeulen (1975).[119] Estradiol and DHEA levels after a single intramuscular injection of Gynodian Depot (4 mg estradiol valerate, 200 mg prasterone enanthate in oil) or Primogyn Depot (10 mg estradiol valerate in oil) in women.[120][121][122] Assays were performed using RIA.[121][122] Sources were Düsterberg & Wendt (1983) and Rauramo et al. (1980).[120][121][122]
Estradiol and DHEA levels after a single intramuscular injection of Gynodian Depot (4 mg estradiol valerate, 200 mg prasterone enanthate in oil) or Primogyn Depot (10 mg estradiol valerate in oil) in women.[120][121][122] Assays were performed using RIA.[121][122] Sources were Düsterberg & Wendt (1983) and Rauramo et al. (1980).[120][121][122]
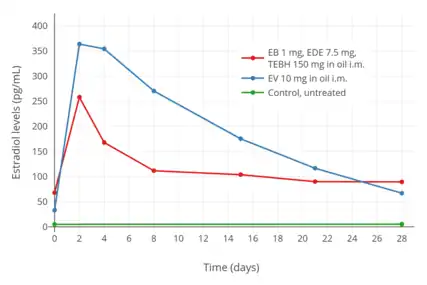 Estradiol levels after an intramuscular injection of 10 mg estradiol valerate in oil, Climacteron (150 mg testosterone enanthate, 1 mg estradiol benzoate, 7.5 mg estradiol dienanthate in oil), and control group in 20, 11, and 11 ovariectomized women, respectively.[125] Assays were performed using RIA.[125] Source was Sherwin et al. (1987).[125]
Estradiol levels after an intramuscular injection of 10 mg estradiol valerate in oil, Climacteron (150 mg testosterone enanthate, 1 mg estradiol benzoate, 7.5 mg estradiol dienanthate in oil), and control group in 20, 11, and 11 ovariectomized women, respectively.[125] Assays were performed using RIA.[125] Source was Sherwin et al. (1987).[125]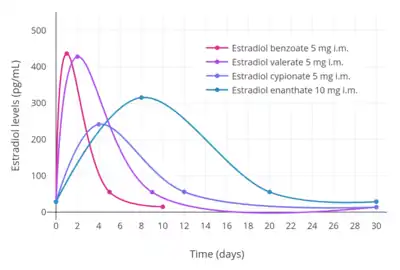
Subcutaneous injection
Estradiol esters like estradiol valerate and estradiol cypionate can be given by subcutaneous injection instead of intramuscular injection.[127][128]
Intravenous injection
The administration of estradiol valerate by intravenous injection has been studied.[3][116] It has been found to be very rapidly cleaved into estradiol.[3][116] The bioavailability and metabolism of estradiol valerate does not differ with intravenous versus intramuscular injection.[116] Conversely, intravenous injection of estradiol valerate has a very short duration, whereas intramuscular injection has a long duration and elimination half-life.[116]
Chemistry

Estradiol valerate is a synthetic estrane steroid and the C17β valerate (pentanoate) fatty acid ester of estradiol.[20][21] It is also known as estradiol 17β-valerate or as estra-1,3,5(10)-triene-3,17β-diol 17β-pentanoate.[20][21] Other common esters of estradiol in use include estradiol cypionate, estradiol enantate, and estradiol acetate, the former two of which are C17β esters of estradiol similarly to estradiol valerate and the latter of which is the C3 acetate ester of estradiol.[20][21]
The experimental octanol/water partition coefficient (logP) of estradiol valerate is 5.6 to 6.3.[129][130]
| Estrogen | Structure | Ester(s) | Relative mol. weight | Relative E2 contentb | logPc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzenecarboxylic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = logP of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
Estradiol valerate was patented by Ciba in 1940 and 1941, with a priority date of 1936.[16][131] It was synthesized and studied, along with a variety of other estradiol esters, by Karl Junkmann of Schering AG in 1953.[132][133] The medication was first introduced for medical use via intramuscular injection in 1954 by Schering in Europe under the brand name Progynon Depot and by Squibb in the United States under the brand name Delestrogen.[17][18][134] In 1966, oral estradiol valerate was introduced by Schering for medical use in Europe under the brand name Progynova.[135][136][137][138][139] A report of its metabolism was published in 1967.[140] Esterification of estradiol, as in estradiol valerate, is thought to improve its metabolic stability with oral administration.[4][3][141] In 1968, micronized preparations of oral estradiol valerate were first introduced under the brand names Progynova 21 and Progynova 21 mite.[135] Along with estradiol benzoate (1933)[142][143][144] and estradiol cypionate (1952),[145] estradiol valerate is one of the most widely used esters of estradiol.[19]
Society and culture
Generic names
Estradiol valerate is the generic name of the drug and its INN, USAN, BANM, and JAN, while oestradiol valerate was formerly its BANM.[20][21][146]
Brand names
Estradiol valerate has been marketed under the brand names Altadiol, Androtardyl-Oestradiol, Ardefem, Climaval, Cyclabil, Cyclocur, Deladiol, Delahormone Unimatic, Delestrogen, Delestrogen 4X, Depogen, Diol-20, Dioval, Ditate, Dura-Estate, Dura-Estradiol, Duratrad, Duragen, Estate, Estra-L, Estradiol Depot, Estraval, Estraval Depot, Estraval PA, Estravel, Femogen, Femogex, Gynogen L.A., Gynokadin, Lastrogen, Menaval, Merimono, Neofollin, Nuvelle, Oestrogynal, Ostrin Depo, Pelanin, Pharlon, Postoval, Primogyna, Primogyn, Primogyn Depot, Progynon, Progynon Depot, Progynova, Repestrogen, Repo-Estra, Reposo-E, Retestrin, Ronfase, Span-Est, Testaval, and Valergen, among others.[20][21][17][147][146] Neofollin is an oil solution of estradiol valerate.[148][149]
Availability
Oral estradiol valerate is used primarily in Europe, under the brand name Progynova.[150] Although oral estradiol valerate was previously available in the United States,[21] it is no longer available in this country except in combination with dienogest as a combined oral contraceptive (under the brand name Natazia).[36] Estradiol valerate by intramuscular injection is available under the brand name Delestrogen in the United States and Canada and under the brand name Progynon Depot in Europe and elsewhere in the world.[36][21]
Research
SH-834 was a combination of 90 mg estradiol valerate and 300 mg gestonorone caproate for weekly intramuscular injection that was developed by Schering in the 1970s.[151][152][153] It was investigated clinically as a treatment for breast cancer and was found to be effective, but was never marketed.[151][154][153]
See also
References
- https://www.drugs.com/cons/estradiol-and-dienogest.html
- Christoph Zink (1 January 1988). Dictionary of Obstetrics and Gynecology. Walter de Gruyter. p. 86. ISBN 978-3-11-085727-6. Retrieved 20 May 2012.
- Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 978-0-323-03309-1.
- Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/S0010-7824(80)80018-7. PMID 7389356.
- Lauritzen, Christian (1988). "Natürliche und Synthetische Sexualhormone – Biologische Grundlagen und Behandlungsprinzipien" [Natural and Synthetic Sexual Hormones – Biological Basis and Medical Treatment Principles]. In Hermann P. G. Schneider; Christian Lauritzen; Eberhard Nieschlag (eds.). Grundlagen und Klinik der Menschlichen Fortpflanzung [Foundations and Clinic of Human Reproduction] (in German). Walter de Gruyter. pp. 229–306. ISBN 978-3110109689. OCLC 35483492.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 551–. ISBN 978-3-642-96158-8.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009402s052lbl.pdf
- Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- Cirigliano M (June 2007). "Bioidentical hormone therapy: a review of the evidence". J Womens Health (Larchmt). 16 (5): 600–31. doi:10.1089/jwh.2006.0311. PMID 17627398.
- Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 419–. ISBN 978-93-5090-575-3.
- A. Kleemann; J. Engel; B. Kutscher; D. Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1167–1174. ISBN 978-3-13-179525-0.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1477–1478. ISBN 978-0-8155-1856-3.
- Larry L. Duetsch (1969). Research and development, market power, and patent policy in ethical drugs. University of Wisconsin--Madison. p. 95.
1954. Delestrogen. Estradiol valerate.
- Samuel S. C. Yen (1991). Reproductive endocrinology: physiology, pathophysiology, and clinical management. Saunders. ISBN 978-0-7216-3206-3. Retrieved 20 May 2012.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 898–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 405. ISBN 978-3-88763-075-1. Retrieved 20 May 2012.
- https://www.drugs.com/availability/generic-delestrogen.html
- Guida M, Bifulco G, Di Spiezio Sardo A, Scala M, Fernandez LM, Nappi C (August 2010). "Review of the safety, efficacy and patient acceptability of the combined dienogest/estradiol valerate contraceptive pill". International Journal of Women's Health. 2: 279–90. doi:10.2147/IJWH.S6954. PMC 2990895. PMID 21151673.
- Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- http://www.wjpps.com/download/article/1412071798.pdf
- Rowlands, S (2009). "New technologies in contraception" (PDF). BJOG: An International Journal of Obstetrics & Gynaecology. 116 (2): 230–239. doi:10.1111/j.1471-0528.2008.01985.x. ISSN 1470-0328. PMID 19076955. S2CID 3415547.
- Wesp LM, Deutsch MB (March 2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". Psychiatr. Clin. North Am. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
- Smith KP, Madison CM, Milne NM (December 2014). "Gonadal suppressive and cross-sex hormone therapy for gender dysphoria in adolescents and adults". Pharmacotherapy. 34 (12): 1282–97. doi:10.1002/phar.1487. PMID 25220381. S2CID 26979177.
- Randi Ettner; Stan Monstrey; Eli Coleman (20 May 2016). Principles of Transgender Medicine and Surgery. Routledge. pp. 216–. ISBN 978-1-317-51460-2.
- Gianna E. Israel; Donald E. Tarver; Joy Diane Shaffer (1 March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 64–. ISBN 978-1-56639-852-7.
- Coelingh Bennink HJ, Verhoeven C, Dutman AE, Thijssen J (January 2017). "The use of high-dose estrogens for the treatment of breast cancer". Maturitas. 95: 11–23. doi:10.1016/j.maturitas.2016.10.010. PMID 27889048.
- Palmieri C, Patten DK, Januszewski A, Zucchini G, Howell SJ (January 2014). "Breast cancer: current and future endocrine therapies". Mol. Cell. Endocrinol. 382 (1): 695–723. doi:10.1016/j.mce.2013.08.001. PMID 23933149. S2CID 3363705.
- American Medical Association. Dept. of Drugs; Council on Drugs (American Medical Association); American Society for Clinical Pharmacology and Therapeutics (1 February 1977). "Estrogens, Progestagens, Oral Contraceptives, and Ovulatory Agents". AMA drug evaluations. Publishing Sciences Group. pp. 540–572. ISBN 978-0-88416-175-2.
Intramuscular: For replacement therapy, (Estradiol, Estradiol Benzoate) 0.5 to 1.5 mg two or three times weekly; (Estradiol Cypionate) 1 to 5 mg weekly for two or three weeks; (Estradiol Dipropionate) 1 to 5 mg every one to two weeks; (Estradiol Valerate) 10 to 40 mg every one to four weeks.
- Horský, Jan; Presl, Jiří (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In J. Horsky; J. Presl (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- George Morris Piersol (1975). The Cyclopedia of Medicine, Surgery, Specialties. F. A. Davis Company.
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
- Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 276, 313, 379, 561, 566. ISBN 978-3-7692-2114-5.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 2153–. ISBN 978-0-7817-1750-2.
- Devroey P, Pados G (1998). "Preparation of endometrium for egg donation". Hum. Reprod. Update. 4 (6): 856–61. doi:10.1093/humupd/4.6.856. PMID 10098476.
Oestradiol valerate and oestradiol in a micronized form are the most widely used oestrogen per os for steroid substitution therapy. Our regimen, as of most other groups [...] is oestradiol valerate (Progynova; Schering, Berlin, Germany) given in various concentrations throughout the cycle [...]. According to Norfolk's protocol, 2 mg of micronized oestradiol valerate are given on cycle days 1–5. [...] In tablet form, micronized oestradiol valerate is also efficiently absorbed [...]
- M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28–November 2, 1984. Springer Science & Business Media. pp. 397, 399. ISBN 978-94-009-4145-8.
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- Tommaso Falcone; William W. Hurd (14 June 2017). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer. pp. 179–. ISBN 978-3-319-52210-4.
- Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 276, 454–455, 566–567. ISBN 978-3-7692-2114-5.
- Krishna; Usha R. And Shah (1996). Menopause. Orient Blackswan. pp. 70–. ISBN 978-81-250-0910-8.
- "NNR: Products Recently Accepted by the A. M. A. Council on Pharmacy and Chemistry". Journal of the American Pharmaceutical Association (Practical Pharmacy ed.). 10 (11): 692–694. 1949. doi:10.1016/S0095-9561(16)31995-8. ISSN 0095-9561.
- http://www.medicines.org.au/files/secaerod.pdf
- https://www.drugs.com/international/estradiol.html
- Sahin FK, Koken G, Cosar E, Arioz DT, Degirmenci B, Albayrak R, Acar M (2008). "Effect of Aerodiol administration on ocular arteries in postmenopausal women". Gynecol. Endocrinol. 24 (4): 173–7. doi:10.1080/09513590701807431. PMID 18382901.
300 μg 17β-estradiol (Aerodiol®; Servier, Chambrayles-Tours, France) was administered via the nasal route by a gynecologist. This product is unavailable after March 31, 2007 because its manufacturing and marketing are being discontinued.
- Leo Jr. Plouffe; Veronica A. Ravnikar; Leon Speroff; Nelson B. Watts (6 December 2012). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 271–. ISBN 978-1-4612-4330-4.
- University of California (1868-1952) (1952). Hospital Formulary and Compendium of Useful Information. University of California Press. pp. 49–. GGKEY:2UAAZRZ5LN0.
- Freimut A. Leidenberger (17 April 2013). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 527–. ISBN 978-3-662-08110-5.
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. pp. 95–98, 488. ISBN 978-0-203-48612-2.
- Laurtizen, Christian (2001). "Hormone Substitution Before, During and After Menopause" (PDF). In Fisch, Franz H. (ed.). Menopause – Andropause: Hormone Replacement Therapy Through the Ages. Krause & Pachernegg: Gablitz. pp. 67–88. ISBN 978-3-901299-34-6.
- Midwinter, Audrey (1976). "Contraindications to estrogen therapy and management of the menopausal syndrome in these cases". In Campbell, Stuart (ed.). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. MTP Press Limited. pp. 377–382. doi:10.1007/978-94-011-6165-7_33. ISBN 978-94-011-6167-1.
- Bishop BM (December 2015). "Pharmacotherapy Considerations in the Management of Transgender Patients: A Brief Review". Pharmacotherapy. 35 (12): 1130–9. doi:10.1002/phar.1668. PMID 26684553. S2CID 37001563.
- Gunther Göretzlehner; Christian Lauritzen; Ulf Göretzlehner (10 December 2008). "Hormontherapie bei gynäkologischen Erkrankungen". Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 245–314. ISBN 978-3-11-020864-1.
Dosierungsbeispiele bei Mammahypoplasie und Infantilismus [...] Parenteral 1. 40 mg Estradiolvalerat (Estradiol-Depot 10 mg JENAPHARM) und 250 mg Hydroxyprogesteroncaproat (Progesteron-Depot JENAPHARM, Proluton Depot) i. m. einmal wöchentlich über 15–20 Wochen lang. 2. 20–40 mg Estradiolvalerat (Estradiol-Depot 10 mg JENAPHARM) i. m. in der ersten und zweiten Woche. 40 mg Estradiolvalerat (Estradiol-Depot 10 mg JENAPHARM) und 250 mg Hydroxyprogesteroncaproat (Progesteron-Depot JENAPHARM, Proluton Depot) i. m. in der dritten und vierten Woche. Therapiedauer 4–5 Monate. Evtl. Abstand zwischen 2 Injektionen auf 2 Wochen erweitern (Abb. 6.2).
- Ulrich U, Pfeifer T, Lauritzen C (1994). "Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report". Horm. Metab. Res. 26 (9): 428–31. doi:10.1055/s-2007-1001723. PMID 7835827.
- Notter G, Kaigas M (September 1966). "Behandlung des inoperablen und metastasierenden Mammakarzinoms mit gestagenen und östrogenen Hormonen" [The treatment of inoperable and metastasizing breast carcinoma with gestational and estrogenic hormones]. Munchener medizinische Wochenschrift (1950) (in German). 108 (39): 1920–1923. ISSN 0027-2973. PMID 6014870.
- Berndt G, Stender HS (November 1970). "Die Östrogen-Gestagen-Kombinationsbehandlung des metastasierenden Mammakarzinoms mit SH 834" [The combined estrogen-gestagen treatment of metastasizing mammary carcinoma using with SH 834]. Dtsch. Med. Wochenschr. (in German). 95 (48): 2399–2404. doi:10.1055/s-0028-1108843. ISSN 0012-0472. PMID 5529652.
- Berndt G, Eckel H, Notter G, Stender HS. "Die Wirkung einer Ostrogen-Gestagen-Kombinationstherapie beim fortgeschrittenen Mammakarzinom mit besonderer Berucksichtigung der Lungenmetastasen" [Effect of combined estrogen-gestagen therapy on advanced breast carcinoma with special consideration of lung metastases]. Strahlentherapie (in German). 141 (5): 540–548. ISSN 0039-2073.
- Notter G, Berndt G (October 1975). "Hormonal treatment of mammary carcinoma with Progynon-Depot and Depostat". Acta Radiol Ther Phys Biol. 14 (5): 433–42. doi:10.3109/02841867509132684. PMID 1202923.
- Firusian N, Schietzel M (September 1976). "Zur additiven Therapie des metastasierenden Mamma-Karzinoms unter Berücksichtigung des Postmenopausalaltes (Ergebnisse einer randomisierten Studie)" [Additive treatment of metastasizing breast cancer with special reference to postmenopausal age (results of a randomized study)]. Strahlentherapie (in German). 152 (3): 235–47. ISSN 0039-2073. PMID 968923.
- Schubert GE, Ziegler H, Völter D (1973). "Vergleichende histologische und zytologische Untersuchungen der Prostata unter besonderer Beruksichtigung oestrogeninduzierter Veranderungen" [Comparison of histological and cytological studies of the prostate with special reference to oestrogene induced changes]. Verhandlungen der Deutschen Gesellschaft für Pathologie (in German). 57: 315–318. ISSN 0070-4113. PMID 4142204.
- Ziegler, H.; Völter, D.; Schubert, G. E. (1974). "Morphological criteria for the control of carcinoma of the prostate with estrogen therapy". International Urology and Nephrology. 6 (3–4): 195–200. doi:10.1007/BF02089265. ISSN 0301-1623. PMID 4142482. S2CID 39028149.
- Harry Benjamin; Gobind Behari Lal; Richard Green; Robert E. L. Masters (1966). The Transsexual Phenomenon. Ace Publishing Company. p. 107.
In my own practice, Squibb's Delestrogen for intramuscular injections was employed with much satisfaction and positive results. This is a slowly absorbing, well-tolerated, potent preparation (chemically, Estradiol Valerate), and was applied in doses of 20 to 60 mg. (½ to 1 ½ cc.). Usually 30 to 60 mg. of Delalutin (Squibb) was added, an equally potent progesterone. This combination was given once a week or once in two to three weeks, according to the response as measured by the patient's emotional balance and physical feminization symptoms. Generally I found that dosage seems less important than length and regularity of administration.
- Benjamin, Harry (1967). "Transvestism and Transsexualism in the male and female1". Journal of Sex Research. 3 (2): 107–127. doi:10.1080/00224496709550519. ISSN 0022-4499.
Estrogen treatment—as already indicated—helps greatly but does not cure. I have employed either Squibb's Delestrogen, a slowly absorbing, highly potent preparation which is, chemically, estradiol valerate (40 mg. to 1 cc); or the still more potent Delestrec, which is estradiol undecylate (100 mg. to 1 cc). This preparation, however, is not yet on the market in this country, though it is widely used in Europe. In the majority of cases, I used from 30 to 100 mg. weekly, or every two to three weeks, by intramuscular injection.
- Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (February 2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacol. Sin. 22 (2): 148–54. PMID 11741520.
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- Lauritzen C (June 1977). "[Estrogen thearpy in practice. 3. Estrogen preparations and combination preparations]" [Estrogen therapy in practice. 3. Estrogen preparations and combination preparations]. Fortschritte Der Medizin (in German). 95 (21): 1388–92. PMID 559617.
- Wolf AS, Schneider HP (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 78–. ISBN 978-3-642-75101-1.
- Göretzlehner G, Lauritzen C, Römer T, Rossmanith W (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 44–. ISBN 978-3-11-024568-4.
- Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl J (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598–599. ISBN 978-3-11-150424-7.
- Lauritzen CH (January 1976). "The female climacteric syndrome: significance, problems, treatment". Acta Obstetricia Et Gynecologica Scandinavica. Supplement. 51: 47–61. doi:10.3109/00016347509156433. PMID 779393.
- Lauritzen C (1975). "The Female Climacteric Syndrome: Significance, Problems, Treatment". Acta Obstetricia et Gynecologica Scandinavica. 54 (s51): 48–61. doi:10.3109/00016347509156433. ISSN 0001-6349.
- Kopera H (1991). "Hormone der Gonaden". Hormonelle Therapie für die Frau. Kliniktaschenbücher. pp. 59–124. doi:10.1007/978-3-642-95670-6_6. ISBN 978-3-540-54554-5. ISSN 0172-777X.
- Scott WW, Menon M, Walsh PC (April 1980). "Hormonal Therapy of Prostatic Cancer". Cancer. 45 Suppl 7: 1929–1936. doi:10.1002/cncr.1980.45.s7.1929. PMID 29603164.
- Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgender Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- Ryden AB (1950). "Natural and synthetic oestrogenic substances; their relative effectiveness when administered orally". Acta Endocrinologica. 4 (2): 121–39. doi:10.1530/acta.0.0040121. PMID 15432047.
- Ryden AB (1951). "The effectiveness of natural and synthetic oestrogenic substances in women". Acta Endocrinologica. 8 (2): 175–91. doi:10.1530/acta.0.0080175. PMID 14902290.
- Kottmeier HL (1947). "Ueber blutungen in der menopause: Speziell der klinischen bedeutung eines endometriums mit zeichen hormonaler beeinflussung: Part I". Acta Obstetricia et Gynecologica Scandinavica. 27 (s6): 1–121. doi:10.3109/00016344709154486. ISSN 0001-6349.
There is no doubt that the conversion of the endometrium with injections of both synthetic and native estrogenic hormone preparations succeeds, but the opinion whether native, orally administered preparations can produce a proliferation mucosa changes with different authors. PEDERSEN-BJERGAARD (1939) was able to show that 90% of the folliculin taken up in the blood of the vena portae is inactivated in the liver. Neither KAUFMANN (1933, 1935), RAUSCHER (1939, 1942) nor HERRNBERGER (1941) succeeded in bringing a castration endometrium into proliferation using large doses of orally administered preparations of estrone or estradiol. Other results are reported by NEUSTAEDTER (1939), LAUTERWEIN (1940) and FERIN (1941); they succeeded in converting an atrophic castration endometrium into an unambiguous proliferation mucosa with 120–300 oestradiol or with 380 oestrone.
- Rietbrock N, Staib AH, Loew D (11 March 2013). Klinische Pharmakologie: Arzneitherapie. Springer-Verlag. pp. 426–. ISBN 978-3-642-57636-2.
- Martinez-Manautou J, Rudel HW (1966). "Antiovulatory Activity of Several Synthetic and Natural Estrogens". In Robert Benjamin Greenblatt (ed.). Ovulation: Stimulation, Suppression, and Detection. Lippincott. pp. 243–253.
- Herr F, Revesz C, Manson AJ, Jewell JB (1970). "Biological Properties of Estrogen Sulfates". Chemical and Biological Aspects of Steroid Conjugation. pp. 368–408. doi:10.1007/978-3-642-49793-3_8. ISBN 978-3-642-49506-9.
- Duncan CJ, Kistner RW, Mansell H (October 1956). "Suppression of ovulation by trip-anisyl chloroethylene (TACE)". Obstetrics and Gynecology. 8 (4): 399–407. PMID 13370006.
- Micks EA, Jensen JT (January 2013). "Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill". Adv Ther. 30 (1): 1–13. doi:10.1007/s12325-012-0071-3. PMID 23239397. S2CID 31125733.
- Hardman SM, Gebbie AE (July 2009). "Hormonal contraceptive regimens in the perimenopause". Maturitas. 63 (3): 204–12. doi:10.1016/j.maturitas.2009.05.001. PMID 19524378.
- Lindberg UB, Crona N, Stigendal L, Teger-Nilsson AC, Silfverstolpe G (February 1989). "A comparison between effects of estradiol valerate and low dose ethinyl estradiol on haemostasis parameters". Thromb. Haemost. 61 (1): 65–9. doi:10.1055/s-0038-1646528. PMID 2526387.
- Wiegratz I, Lee JH, Kutschera E, Winkler UH, Kuhl H (August 2004). "Effect of four oral contraceptives on hemostatic parameters". Contraception. 70 (2): 97–106. doi:10.1016/j.contraception.2004.03.004. PMID 15288212.
- Trémollieres F (February 2012). "Contraception orale estro-progestative: quelle différence entre éthinylestradiol et estradiol?" [Oral combined contraception: is there any difference between ethinyl-estradiol and estradiol?]. Gynecol Obstet Fertil (in French). 40 (2): 109–15. doi:10.1016/j.gyobfe.2011.10.009. PMID 22244780.
- L'Hermite M (September 1990). "Risks of estrogens and progestogens". Maturitas. 12 (3): 215–46. doi:10.1016/0378-5122(90)90005-q. PMID 2170823.
- Ottosson UB, Carlström K, Johansson BG, von Schoultz B (1986). "Estrogen induction of liver proteins and high-density lipoprotein cholesterol: comparison between estradiol valerate and ethinyl estradiol". Gynecol. Obstet. Invest. 22 (4): 198–205. doi:10.1159/000298914. PMID 3817605.
- Fåhraeus L (November 1988). "The effects of estradiol on blood lipids and lipoproteins in postmenopausal women". Obstet Gynecol. 72 (5 Suppl): 18S–22S. PMID 3173937.
- Sitruk-Ware R, Nath A (June 2011). "Metabolic effects of contraceptive steroids". Rev Endocr Metab Disord. 12 (2): 63–75. doi:10.1007/s11154-011-9182-4. PMID 21538049. S2CID 23760705.
- Fruzzetti F, Trémollieres F, Bitzer J (May 2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- Mueller A, Dittrich R, Binder H, Kuehnel W, Maltaris T, Hoffmann I, Beckmann MW (July 2005). "High dose estrogen treatment increases bone mineral density in male-to-female transsexuals receiving gonadotropin-releasing hormone agonist in the absence of testosterone". Eur. J. Endocrinol. 153 (1): 107–13. doi:10.1530/eje.1.01943. PMID 15994752.
- Mueller A, Binder H, Cupisti S, Hoffmann I, Beckmann MW, Dittrich R (March 2006). "Effects on the male endocrine system of long-term treatment with gonadotropin-releasing hormone agonists and estrogens in male-to-female transsexuals". Horm. Metab. Res. 38 (3): 183–7. doi:10.1055/s-2006-925198. PMID 16673210.
- Odlind V, Milsom I, Persson I, Victor A (June 2002). "Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills?". Acta Obstet Gynecol Scand. 81 (6): 482–90. doi:10.1034/j.1600-0412.2002.810603.x. PMID 12047300. S2CID 26054257.
- "Progynova 1mg (SPC) | Drugs.com". Retrieved 2012-09-06.
- Shellenberger, T. E. (1986). "Pharmacology of estrogens". The Climacteric in Perspective. pp. 393–410. doi:10.1007/978-94-009-4145-8_36. ISBN 978-94-010-8339-3.
- O'Connell MB (1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9 Suppl): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713. S2CID 10159196.
- Wiegratz I, Fink T, Rohr UD, Lang E, Leukel P, Kuhl H (September 2001). "Überkreuz-Vergleich der Pharmakokinetik von Estradiol unter der Hormonsubstitution mit Estradiolvalerat oder mikronisiertem Estradiol" [Cross-over comparison of the pharmacokinetics of estradiol during hormone replacement therapy with estradiol valerate or micronized estradiol]. Zentralbl Gynakol (in German). 123 (9): 505–12. doi:10.1055/s-2001-18223. PMID 11709743.
- Fruzzetti F, Bitzer J (2010). "Review of clinical experience with estradiol in combined oral contraceptives". Contraception. 81 (1): 8–15. doi:10.1016/j.contraception.2009.08.010. PMID 20004267.
- Vree TB, Timmer CJ (August 1998). "Enterohepatic cycling and pharmacokinetics of oestradiol in postmenopausal women". J. Pharm. Pharmacol. 50 (8): 857–64. doi:10.1111/j.2042-7158.1998.tb04000.x. PMID 9751449. S2CID 23550553.
- Timmer CJ, Geurts TB (1999). "Bioequivalence assessment of three different estradiol formulations in postmenopausal women in an open, randomized, single-dose, 3-way cross-over study". Eur J Drug Metab Pharmacokinet. 24 (1): 47–53. doi:10.1007/BF03190010. PMID 10412891. S2CID 20513936.
- Jensen, Jeffrey; Bitzer, Johannes; Serrani, Marco (2013). "Comparison of the pharmacologic and clinical profiles of new combined oral contraceptives containing estradiol". Open Access Journal of Contraception: 39. doi:10.2147/OAJC.S50693. ISSN 1179-1527.
- Serhal PF, Craft IL (May 1989). "Oocyte donation in 61 patients". Lancet. 1 (8648): 1185–7. doi:10.1016/S0140-6736(89)92762-1. PMID 2566746. S2CID 21953983.
- Serhal P (July 1990). "Oocyte donation and surrogacy". Br. Med. Bull. 46 (3): 796–812. doi:10.1093/oxfordjournals.bmb.a072432. PMID 2207608.
- Lim HH, Jang YH, Choi GY, Lee JJ, Lee ES (January 2019). "Gender affirmative care of transgender people: a single center's experience in Korea". Obstet Gynecol Sci. 62 (1): 46–55. doi:10.5468/ogs.2019.62.1.46. PMC 6333764. PMID 30671393.
When we prescribed estradiol, we preferred sublingual estradiol valerate instead of the oral form for feminizing HT since prior researchers have reported the effectiveness of sublingual administration in maintaining high blood estradiol concentration and low E1/E2 ratio [13].
- Pines A, Averbuch M, Fisman EZ, Rosano GM (September 1999). "The acute effects of sublingual 17beta-estradiol on the cardiovascular system". Maturitas. 33 (1): 81–5. doi:10.1016/S0378-5122(99)00036-5. PMID 10585176.
- Sriram (2007). Medicinal Chemistry. Pearson Education India. p. 427. ISBN 978-81-317-0031-0. Retrieved 20 May 2012.
- Düsterberg B, Schmidt-Gollwitzer M, Hümpel M (1985). "Pharmacokinetics and biotransformation of estradiol valerate in ovariectomized women". Horm. Res. 21 (3): 145–54. doi:10.1159/000180039. PMID 2987096.
- Schug BS, Donath F, Blume HH (February 2012). "Bioavailability and pharmacodynamics of two 10-mg estradiol valerate depot formulations following IM single dose administration in healthy postmenopausal volunteers". Int J Clin Pharmacol Ther. 50 (2): 100–17. doi:10.5414/CP201589. PMID 22257576.
- Göretzlehner G, Ackermann W, Angelow K, Bergmann G, Bieck E, Golbs S, Kliem O (2002). "Pharmakokinetik von Estron, Estradiol, FSH, LH und Prolaktin nach intramuskulärer Applikation von 5 mg Estradiolvalerat" [Pharmacokinetics of estradiol valerate in postmenopausal women after intramuscular administration]. Journal für Menopause. 9 (2): 51–55.
- Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- Kuhl, Herbert; Taubert, Hans-Dieter (1987). Das Klimakterium – Pathophysiologie, Klinik, Therapie [The Climacteric – Pathophysiology, Clinic, Therapy] (in German). Stuttgart, Germany: Thieme Verlag. p. 122. ISBN 978-3137008019.
- Düsterberg B, Wendt H (1983). "Plasma levels of dehydroepiandrosterone and 17 beta-estradiol after intramuscular administration of Gynodian-Depot in 3 women". Horm. Res. 17 (2): 84–9. doi:10.1159/000179680. PMID 6220949.
- Rauramo L, Punnonen R, Kaihola LH, Grönroos M (January 1980). "Serum oestrone, oestradiol and oestriol concentrations in castrated women during intramuscular oestradiol valerate and oestradiolbenzoate-oestradiolphenylpropionate therapy". Maturitas. 2 (1): 53–8. doi:10.1016/0378-5122(80)90060-2. PMID 7402086.
- Gerhard Geppert (1975). Untersuchungen zur Pharmakokinetik von Östradiol-17β, Östradiol-Benzoat, Östradiol-Valerianat und Östradiol-Undezylat bei der Frau: der Verlauf der Konzentrationen von Östradiol-17β, Östron, LH und FSH im Serum [Studies on the pharmacokinetics of estradiol-17β, estradiol benzoate, estradiol valerate, and estradiol undecylate in women: the progression of serum estradiol-17β, estrone, LH, and FSH concentrations]. pp. 1–34. OCLC 632312599.
- Leyendecker G, Geppert G, Nocke W, Ufer J (May 1975). "Untersuchungen zur Pharmakokinetik von Östradiol-17β, Östradiol-benzoat, Östradiol-Valerianat un Östradiol-Undezylat bei der Frau: Der Verlauf der Konzentration von Östradiol-17β, Östron, LH und FSH im Serum" [Estradiol 17β, estrone, LH and FSH in serum after administration of estradiol 17β, estradiol benzoate, estradiol valeriate and estradiol undecylate in the female]. Geburtshilfe Frauenheilkd (in German). 35 (5): 370–374. ISSN 0016-5751. PMID 1150068.
- Sherwin BB, Gelfand MM, Schucher R, Gabor J (February 1987). "Postmenopausal estrogen and androgen replacement and lipoprotein lipid concentrations". Am. J. Obstet. Gynecol. 156 (2): 414–9. doi:10.1016/0002-9378(87)90295-x. PMID 3826177.
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Unger CA (December 2016). "Hormone therapy for transgender patients". Transl Androl Urol. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
- Sierra-Ramírez JA, Lara-Ricalde R, Lujan M, Velázquez-Ramírez N, Godínez-Victoria M, Hernádez-Munguía IA, Padilla A, Garza-Flores J (2011). "Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg)". Contraception. 84 (6): 565–70. doi:10.1016/j.contraception.2011.03.014. PMID 22078184.
- Corwin Hansch; Peter George Sammes; John Bodenhan Taylor (1990). Comprehensive Medicinal Chemistry: Cumulative subject index & drug compendium. Elsevier Science Limited. ISBN 978-0-08-037062-0.
- http://www.chemspider.com/Chemical-Structure.13194.html
- https://patents.google.com/patent/US2205627A/en
- Shoham Z, Kopernik G (June 2004). "Tools for making correct decisions regarding hormone therapy. part I: background and drugs". Fertil. Steril. 81 (6): 1447–57. doi:10.1016/j.fertnstert.2003.10.052. PMID 15193460.
- Junkmann, Karl (1953). "Über protrahiert wirksame Östrogene" [Over protracted effective estrogens]. Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie. 220 (5). doi:10.1007/BF00246561. ISSN 0028-1298. S2CID 20753905.
- Ehrengut, Wolfgang (1954). "Über ovarielle Agenesie". Zeitschrift für Kinderheilkunde. 75 (3): 224–234. doi:10.1007/BF00439822. ISSN 0340-6199. S2CID 29364660.
Um die "Menarche" sollte eine verstärkte Substitutionstherapie (20 Tage lang tgl. 0,1 mg Follikelhormon per os oder einmalig Progynon-Depot (10 mg i.m.), [...]
- Kuhl, Herbert; Wiegratz, Inka (1 January 2008). Klimakterium, Postmenopause und Hormonsubstitution [Climacteric, Postmenopause and Hormone Replacement] (in German) (4 ed.). UNI-MED-Verlag. p. 18. ISBN 978-3-83742-043-2.
With Progynon Depot-10, an oily solution of 10 mg estradiol valerate, an injection preparation had been available since 1953 and since 1966 coated tablets with estradiol valerate for oral therapy. The first Schering preparation containing micronized estradiol was marketed in 1968 as Progynova 21 (2 mg) and Progynova 21 mite (1 mg).
- "Neue Spezialitäten". Klinische Wochenschrift. 44 (23): 1381. 1966. doi:10.1007/BF01747900. ISSN 0023-2173. S2CID 20357182.
NEUE SPEZIALITATEN [...] Progynova. 1 Dragee enthält 2 mg Oestradiolvalerinat (Klimakterium). Hersteller: Schering AG, Berlin 65.
- Dapunt O (September 1967). "Behandlung klimakterischer Beschwerden mit Östradiolvalerianat (Progynova)" [The management of climacteric disorders using estradiol valerate (Progynova)]. Med Klin (in German). 62 (35): 1356–61 passim. ISSN 0025-8458. PMID 5593020.
- Velikay L (March 1968). "Die perorale Behandlung des klimakterischen Syndroms mit Ostradiolvalerianat" [The peroral treatment of the climacteric syndrome with estradiol valerate]. Wien. Klin. Wochenschr. (in German). 80 (12): 229–33. ISSN 0043-5325. PMID 5728263.
- Koed J (May 1972). "Zur Behandlung klimakterischer Ausfallserscheinungen mit Progynova" [Therapy of climacteric deficiency symptoms using Progynova]. Med Welt (in German). 23 (22): 834–6. ISSN 0025-8512. PMID 5045321.
- Kolb KH (1967). "The metabolism of oestradiol valerate". Medizinische Mitteilungen (Schering). 28: 16–. ISSN 0301-2492.
- http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Set_Current_Drug&ApplNo=084499&DrugName=ESTRACE&ActiveIngred=ESTRADIOL&SponsorApplicant=BRISTOL%20MYERS%20SQUIBB&ProductMktStatus=3&goto=Search.DrugDetails
- Kaufman, C. (1933). "Die Behandlung der Amenorrhöe mit Hohen Dosen der Ovarialhormone". Klinische Wochenschrift. 12 (40): 1557–1562. doi:10.1007/BF01765673. ISSN 0023-2173. S2CID 25856898.
- Buschbeck, Herbert (2009). "Neue Wege der Hormontherapie in der Gynäkologie" [New ways of hormonal therapy in gynecology]. Deutsche Medizinische Wochenschrift. 60 (11): 389–393. doi:10.1055/s-0028-1129842. ISSN 0012-0472.
- Biskind, Morton S. (1935). "Commercial Glandular Products". Journal of the American Medical Association. 105 (9): 667. doi:10.1001/jama.1935.92760350007009a. ISSN 0002-9955.
Progynon-B, Schering Corporation: This is crystalline hydroxyestrin benzoate obtained by hydrogenation of theelin and subsequent conversion to the benzoate. [...] Progynon-B is marketed in ampules containing 1 cc. of a sesame oil solution of hydroxyestrin benzoate of either 2,500, 5,000, 10,000 or 50,000 international units.
- Marshall Sittig (1 January 1988). Pharmaceutical Manufacturing Encyclopedia. William Andrew. pp. 575–576. ISBN 978-0-8155-1144-1. Retrieved 20 May 2012.
- https://www.drugs.com/international/estradiol.html
- Richard J. Lewis (13 June 2008). Hazardous Chemicals Desk Reference. John Wiley & Sons. pp. 594–. ISBN 978-0-470-18024-2.
- Kubíková, Drahomíra (2014). "Menopauzální symptomy a hormonální substituční terapie" [Menopausal symptoms and hormone replacement therapy]. Praktické Lékárenství (in Czech). 10 (2): 68–73. ISSN 1801-2434.
- http://www.sukl.cz/download/pil/PI16359.pdf
- Joseph S. Sanfilippo (January 1998). Primary Care in Obstetrics and Gynecology: A Handbook for Clinicians. Springer Science & Business Media. pp. 227–. ISBN 978-0-387-94739-6.
- Notter, G.; Berndt, G. (2009). "Hormonal Treatment of Mammary Carcinoma with Progynon-Depot and Depostat". Acta Radiologica: Therapy, Physics, Biology. 14 (5): 433–442. doi:10.3109/02841867509132684. ISSN 0567-8064. PMID 1202923.
- Ward, H. W. C. (1972). "Progestogen Therapy for Ovarian Carcinoma". BJOG: An International Journal of Obstetrics and Gynaecology. 79 (6): 555–559. doi:10.1111/j.1471-0528.1972.tb14200.x. ISSN 1470-0328. PMID 4555897. S2CID 2586346.
- Berndt G, Eckel H, Notter G, St Stender H (May 1971). "Die Wirkung einer Ostrogen-Gestagen-Kombinationstherapie beim fortgeschrittenen Mammakarzinom mit besonderer Berucksichtigung der Lungenmetastasen" [Effect of Estrogen-Gestagen Combination Therapy in Advanced Breast Carcinoma with Special Reference to Pulmonary Metastases]. Strahlentherapie (in German). 141 (5): 540–8. PMID 5088730.
- Berndt, G.; Stender, H.-St. (2009). "[The combined estrogen-gestagen treatment of metastasizing mammary carcinoma using with SH 834]". Deutsche Medizinische Wochenschrift (1946). 95 (48): 2399–2404. doi:10.1055/s-0028-1108843. ISSN 0012-0472. PMID 5529652.
Further reading
- Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- Sang GW (1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- O'Connell MB (1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9 Suppl): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713. S2CID 10159196.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.