Ethanethiol
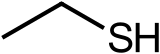
Ethanethiol, commonly known as ethyl mercaptan and stench,[5] is a clear liquid with a distinct odor. It is an organosulfur compound with the formula CH3CH2SH. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with sulfur in place of oxygen. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic. It occurs naturally as a minor component of petroleum, and may be added to otherwise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Ethanethiol | |
| Other names
Ethyl mercaptan Mercaptoethane Ethyl sulfhydrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.762 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2363 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H6S | |
| Molar mass | 62.13404 g·mol−1 |
| Appearance | Colorless liquid[3] |
| Odor | Rotten cabbage, flatulence, skunk-like[3] |
| Density | 0.8617 g·cm−3 |
| Melting point | −148 °C (−234 °F; 125 K) |
| Boiling point | 35 °C (95 °F; 308 K) |
| 0.7% (20 °C)[3] | |
| Vapor pressure | 442 mmHg (20 °C)[3] |
| Acidity (pKa) | 10.6 |
| −47.0×10−6 cm3/mol | |
| Hazards | |
| Main hazards | Nauseating |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R11, R20, R50/53 |
| S-phrases (outdated) | S16, S25, S60, S61 |
| NFPA 704 (fire diamond) | |
| Flash point | −48 °C; −55 °F; 225 K[3] |
| Explosive limits | 2.8–18.0%[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
682 mg/kg (rat, oral)[4] |
LC50 (median concentration) |
4410 ppm (rat, 4 hr) 2770 (mouse, 4 hr)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
C 10 ppm (25 mg/m3)[3] |
REL (Recommended) |
C 0.5 ppm (1.3 mg/m3) [15-minute][3] |
IDLH (Immediate danger) |
500 ppm[3] |
| Related compounds | |
Related compounds |
Methanethiol Butanethiol Ethanol thiophenol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Ethanethiol is prepared by the reaction of ethylene with hydrogen sulfide over a catalyst. The various producers utilize different catalysts in this process. It has also been prepared commercially by the reaction of ethanol with hydrogen sulfide gas over an acidic solid catalyst, such as alumina.[6]
Ethanethiol was originally reported by Zeise in 1834.[7] Zeise treated calcium ethyl sulfate with a suspension of barium sulfide saturated with hydrogen sulfide. He is credited with naming the C2H5S- group as mercaptum.
Ethanethiol can also be prepared by a halide displacement reaction, where ethyl halide is treated with aqueous sodium bisulfide. This conversion was demonstrated as early as 1840 by Henri Victor Regnault.[8]
Odor
Ethanethiol has a strongly disagreeable odor that humans can detect in minute concentrations. The threshold for human detection is as low as one part in 2.8 billion parts of air (0.36 parts per billion). Its odor resembles that of leeks, onions, durian or cooked cabbage, but is quite distinct.[9]
Employees of the Union Oil Company of California reported first in 1938 that turkey vultures would gather at the site of any gas leak. After finding that this was caused by traces of ethanethiol in the gas it was decided to boost the amount of ethanethiol in the gas, to make detections of leaks easier. [10][11]
Uses
Ethanethiol is intentionally added to butane and propane (see: LPG) to impart an easily noticed smell to these normally odorless fuels that pose the threat of fire, explosion, and asphyxiation.
In the underground mining industry, ethanethiol or ethyl mercaptan is referred to as "stench gas".[12] The gas is released into mine ventilation systems to alert mine workers during an emergency. In Ontario, mining legislation dictates that "The alarm system in an underground mine shall, consist of the introduction into all workplaces of sufficient quantities of ethyl mercaptan gas or similar gas to be readily detectable by all workers".[13]
Reactions
Ethanethiol is a reagent in organic synthesis. In the presence of sodium hydroxide, it gives the powerful nucleophile EtS−. The salt can be generated quantitatively by reaction with sodium hydride.[14]
Ethanethiol can be oxidized to ethyl sulfonic acid, using strong oxidizing agents. Weaker oxidants, such as ferric oxide or hydrogen peroxide give the disulfide, diethyl disulfide:
- 2 EtSH + H2O2 → EtS-SEt + 2 H2O
Like other thiols, it behaves comparably to hydrogen sulfide. For example, it binds, concomitant with deprotonation to "soft" transition metal cations, such as Hg2+, Cu+, and Ni2+ to give polymeric thiolato complexes, Hg(SEt)2, CuSEt, and Ni(SEt)2, respectively.
See also
- tert-Butylthiol (tert-butyl mercaptan)
- Butanethiol (butyl mercaptan)
References
- Merck Index, 12th edition, hEllon and 3771
- "ICSC 0470 - ETHANETHIOL".
- NIOSH Pocket Guide to Chemical Hazards. "#0280". National Institute for Occupational Safety and Health (NIOSH).
- "Ethyl mercaptan". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Staley, Dennis; Wilbraham, Antony; Matta, Michael; Waterman, Edward (2017). Pearson Chemistry. United States: Pearson Education, Inc. pp. R25. ISBN 978-1-32-320590-7.
- Norell, John; Louthan, Rector P. (1988). "Thiols". Kirk-Othmer Concise Encylclopedia of Chemical Technology (3rd ed.). New York: John Wiley & Sons, Inc. pp. 946–963. ISBN 978-0471801047.
- Zeise, William Christopher (1834). "Sur le Mercaptan; avec des Observations sur d'autres produits resultant de l'action des sulfovinates ainsi que de l'huile de vin, sur des sulfures metalliques" [On mercaptan; with comments on other products resulting from the action of [salts of] ethyl hydrogen sulfate as well as oil of wine [diethyl sulfate] on metallic sulfides]. Annales de Chimie et de Physique. 2nd series (in French). 56: 87–97.
- Regnault, V (1840). "Ueber die Einwirkung des Chlors auf die Chlorwasserstoffäther des Alkohols und Holzgeistes und über mehrere Punkte der Aethertheorie" [On the effect of chlorine on the volatile hydrochlorides of ethanol and methanol and on several points of ether theory]. Annalen der Chemie und Pharmacie (in German). 34: 24–52. doi:10.1002/jlac.18400340103. From p. 24: "Das Aethylsulfür war bis jetzt noch nicht dargestellt worden. Man erhält es sehr leicht durch wechselseitige Zersetzung, wenn man Aethylchlorür mit einer weingeistigen Auflösung von einfach Schwefelkalium zusammenbringt." (Ethanethiol still has not been prepared – until now. One obtains it very easily by reciprocal decomposition [i.e., salt metathesis reaction ], if one brings together ethyl chloride with a solution, in ethanol, of simple potassium hydrogen sulfide.)
- "Ethanethiol price,buy Ethanethiol - chemicalbook". www.chemicalbook.com. Retrieved 16 November 2019.
- Gooley, Tristan (21 May 2015). The Walker's Guide to Outdoor Signs. Sceptre. p. 242. ISBN 9781444780109.
- Nicholls, Henry. "The truth about vultures". Retrieved 2016-10-21.
- "Stench Gas". Zacon Ltd. Archived from the original on 3 April 2015. Retrieved 20 February 2015.
- "Occupational Health and Safety Act: R.R.O. 1990, REGULATION 854 MINES AND MINING PLANTS Sect. 26(6)(a)". Ontario Ministry of Labour. Retrieved 20 February 2015.
- Mirrington, R. N.; Feutrill, G. I. (1988). "Orcinol Monomethyl Ether". Organic Syntheses.; Collective Volume, 6, p. 859
